Question
Question: Maximum kinetic energy \( {E_k} \) of a photoelectron varies with this frequency ( \( \nu \) ) of th...
Maximum kinetic energy Ek of a photoelectron varies with this frequency ( ν ) of the incident radiation as:
(A) 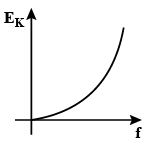
(B) 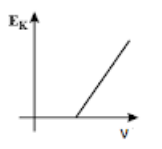
(C) 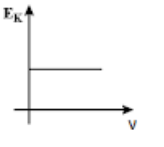
(D) 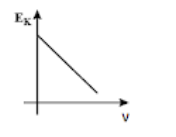
Solution
Hint : In order to solve this question, we are going to first define what the photoelectric effect is and what the relation between the maximum kinetic energy and the frequency is according to Einstein's equation. After that, the correct shape of the curve can be chosen easily.
The maximum kinetic energy Ek of the photoelectrons is given by:
Ek=hν−ϕ where ν is the frequency of the incident radiation and ϕ is the work function of the metal surface.
Complete Step By Step Answer:
The photoelectric effect is a phenomenon where electrons are emitted from the metal surface when the light of suitable frequency falls on it. The suitable frequency is decided on the basis of the threshold frequency, which is the minimum frequency below which the photoelectric effect doesn’t take place.
According to the Einstein’s equation, we can see that the maximum kinetic energy Ek of the photoelectrons is given by:
Ek=hν−ϕ where ν is the frequency of the incident radiation and ϕ is the work function of the metal surface.
This equation matches with the equation for a straight line, y=mx+c , where m gives the slope of the straight line.
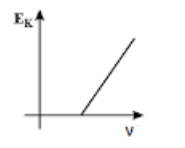
Thus, in the maximum kinetic energy versus the frequency curve, we get the curve in the form of a straight line with a slope h and the y−intercept equal to −ϕ on negative y−axis as shown in option (B).
Note :
The entire energy of the photon is transferred to the electron. A part of this energy is used to remove the electron from the grasp of the metal atom and the rest is given to the ejected electron as kinetic energy. Electrons emitted from underneath the metal surface lose some kinetic energy during the collision. But the surface electrons carry all the kinetic energy imparted by the photon and have the maximum kinetic energy.
