Question
Question: Maximum heat Is released In mixing of...
Maximum heat Is released In mixing of
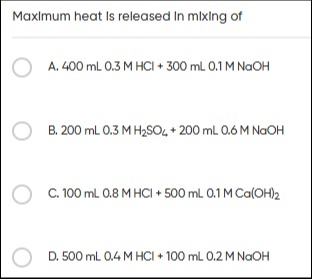
400 mL 0.3 M HCI + 300 mL 0.1 M NaOH
200 mL 0.3 M H2SO4 + 200 mL 0.6 M NaOH
100 mL 0.8 M HCI + 500 mL 0.1 M Ca(OH)2
500 mL 0.4 M HCI + 100 mL 0.2 M NaOH
B. 200 mL 0.3 M H2SO4 + 200 mL 0.6 M NaOH
Solution
The heat released during the neutralization of a strong acid and a strong base is approximately constant per mole of water formed (around -57.3 kJ/mol). Therefore, maximum heat release corresponds to the maximum moles of water formed. We calculate the moles of H+ ions from the acid and OH− ions from the base for each option. The number of moles of water formed is determined by the limiting reactant, which is the minimum of the moles of H+ and OH−.
- Option A: Moles of H+ from HCl = 0.4 L×0.3 M=0.12 mol. Moles of OH− from NaOH = 0.3 L×0.1 M=0.03 mol. Moles of H2O formed = min(0.12,0.03)=0.03 mol.
- Option B: Moles of H+ from H2SO4 = 0.2 L×0.3 M×2=0.12 mol. Moles of OH− from NaOH = 0.2 L×0.6 M=0.12 mol. Moles of H2O formed = min(0.12,0.12)=0.12 mol.
- Option C: Moles of H+ from HCl = 0.1 L×0.8 M=0.08 mol. Moles of OH− from Ca(OH)2 = 0.5 L×0.1 M×2=0.10 mol. Moles of H2O formed = min(0.08,0.10)=0.08 mol.
- Option D: Moles of H+ from HCl = 0.5 L×0.4 M=0.20 mol. Moles of OH− from NaOH = 0.1 L×0.2 M=0.02 mol. Moles of H2O formed = min(0.20,0.02)=0.02 mol.
Comparing the moles of water formed (0.03 mol, 0.12 mol, 0.08 mol, 0.02 mol), Option B yields the maximum moles of water (0.12 mol), and hence the maximum heat release.
