Question
Question: Maximum covalency of Sulphur is: (A) 2 (B) 4 (C) 6 (D) 8...
Maximum covalency of Sulphur is:
(A) 2
(B) 4
(C) 6
(D) 8
Solution
Element can show maximum valency equal to its group number. The valence electrons in sulphur enter the 3rd orbital that is n = 3. Covalency is directly related to the number of unpaired valence electrons.
Complete step by step answer:
Covalent bond is the bond formed by sharing of electrons. The electron pairs shared are called shared pairs or bonding pairs. In the case of covalent bonds the attractive and repulsive forces are balanced between the atoms. An atom forms a covalent bond when the ionization energy of that atom is very high and along with that it has low electron affinity. When an atom has higher ionization enthalpy than it requires a large amount of energy to remove an electron and get converted to positively charged species. If the atom has low electron affinity which refers to the capacity of an atom to accept an electron then in that case that atom could not accept an electron to get converted to negatively charged species.
Covalent bond is when two unpaired electrons of two atoms respectively combine with each other. So more the number of unpaired electrons more covalent bonds will be formed. Covalency is the number of electrons shared by the atom with another atom of the same element or different element to acquire stable configuration. For example, if the number of electrons shared between two atoms is 2 then the covalency is two.
Now, maximum covalency is observed when the electrons are shared between atoms of same electronegativities. Electronegativity is the capacity of the atom to pull the shared pair of electrons towards itself.
Sulphur is an element of group 16 which is an oxygen family. The electronic configuration of Sulphur is 1s22s22p63s23p4. It has 6 valence electrons out of which there are 2 paired electrons and 2 unpaired electrons. Since it has only 2 unpaired electrons it should form only two covalent bonds. But sulphur has vacant d orbitals so there will be excitation of electrons.
Transfer of electrons takes place as follows:
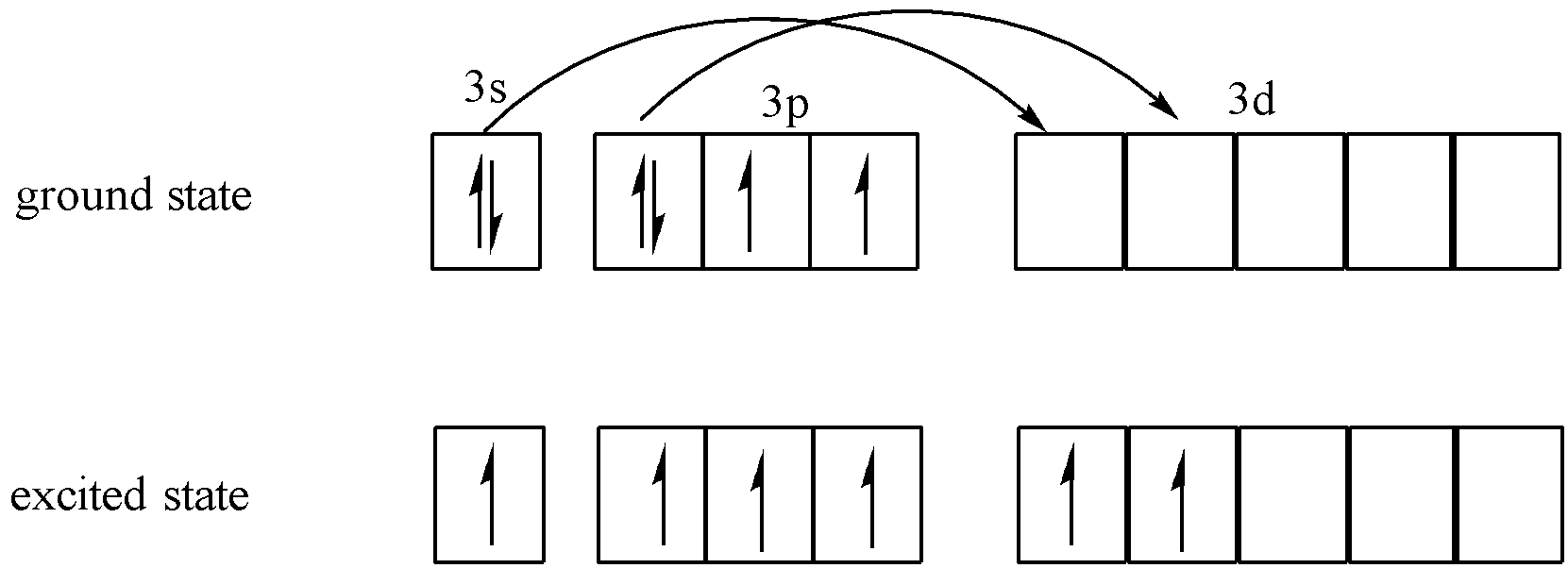
Since the total number of unpaired electrons are 6 therefore the maximum number of covalent bonds that can be formed are 6. Maximum covalency of Sulphur is 6.
So, the correct answer is “Option C”.
Additional Information:
The name sulphur has been derived from Sanskrit word Sulvere which means killer of copper. Sulphur is obtained both in free and combined state in nature.
Note: The maximum covalency of the first element of every group is less as compared to rest elements of that group; this is because of absence of d orbitals, small size and high electronegativity. Similarly oxygen from the group 16 shows maximum covalency of 2 as it does not consist of vacant d orbitals. The hybridization of the compound or molecule can be found by the number of covalent bonds formed. The hybridization of sulphur with maximum valency 6 is sp3d2.
