Question
Question: $\mathrm{VO}+\mathrm{Fe}_{2} \mathrm{O}_{3} \rightarrow \mathrm{FeO}+\mathrm{V}_{2} \mathrm{O}_{5}$...
VO+Fe2O3→FeO+V2O5
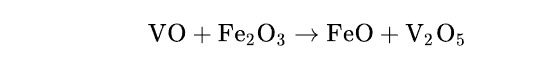
Answer
The balanced chemical equation is:
2VO+3Fe2O3→6FeO+V2O5
Explanation
Solution
The given unbalanced equation VO+Fe2O3→FeO+V2O5 is balanced by ensuring the number of atoms of each element is equal on both the reactant and product sides. By assigning stoichiometric coefficients and solving the resulting system of linear equations for the atoms of V, Fe, and O, the coefficients are found to be 2 for VO, 3 for Fe2O3, 6 for FeO, and 1 for V2O5.
