Question
Question: Match the solutions given in List-I and the colours given in List-II. | | List-I (Aqueous sol...
Match the solutions given in List-I and the colours given in List-II.
| List-I (Aqueous solution of salt) | List-II (Colour) | ||
|---|---|---|---|
| A. | FeSO₄.7H₂O | P. | Green |
| B. | NiCl₂.4H₂O | Q. | Light pink |
| C. | MnCl₂.4H₂O | R. | Blue |
| D. | CoCl₂.6H₂O | S. | Pale green |
| E. | Cu₂Cl₂ | T. | Pink |
| U. | Colourless |
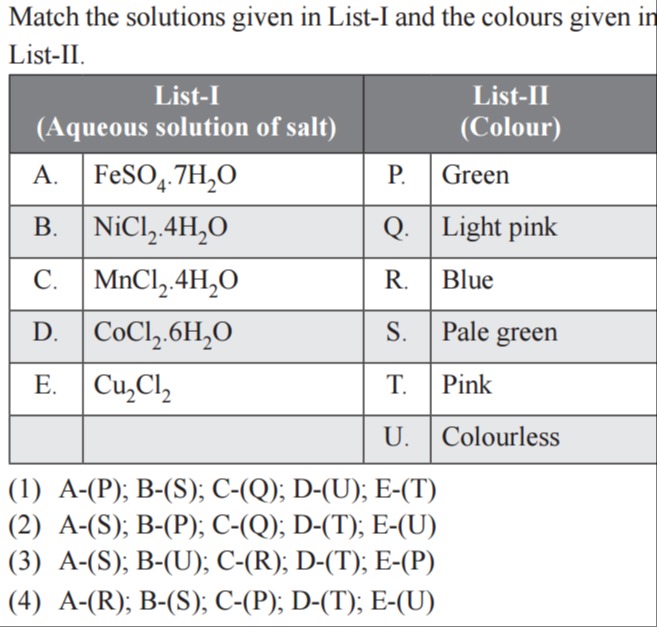
A-(P); B-(S); C-(Q); D-(U); E-(T)
A-(S); B-(P); C-(Q); D-(T); E-(U)
A-(S); B-(U); C-(R); D-(T); E-(P)
A-(R); B-(S); C-(P); D-(T); E-(U)
A-(S); B-(P); C-(Q); D-(T); E-(U)
Solution
The colors of transition metal ions in aqueous solutions are primarily due to d-d transitions, where electrons absorb light in the visible region and get promoted to higher energy d-orbitals. The observed color is the complementary color of the absorbed light. Ions with d⁰ or d¹⁰ configurations are typically colorless as they do not have d-d transitions.
Let's analyze each salt:
A. FeSO₄.7H₂O (Ferrous sulfate heptahydrate)
- This salt contains Fe²⁺ ions.
- Aqueous solutions of Fe²⁺ are typically pale green.
- Therefore, A matches with S.
B. NiCl₂.4H₂O (Nickel(II) chloride tetrahydrate)
- This salt contains Ni²⁺ ions.
- Aqueous solutions of Ni²⁺ are typically green.
- Therefore, B matches with P.
C. MnCl₂.4H₂O (Manganese(II) chloride tetrahydrate)
- This salt contains Mn²⁺ ions.
- Aqueous solutions of Mn²⁺ are typically very light pink or pale pink.
- Therefore, C matches with Q.
D. CoCl₂.6H₂O (Cobalt(II) chloride hexahydrate)
- This salt contains Co²⁺ ions.
- Aqueous solutions of Co²⁺ are typically pink (or rose).
- Therefore, D matches with T.
E. Cu₂Cl₂ (Copper(I) chloride)
- This salt contains Cu⁺ ions.
- The electronic configuration of Cu⁺ is [Ar] 3d¹⁰.
- Since the d-orbital is completely filled (d¹⁰ configuration), there are no d-d transitions possible in the visible region.
- Therefore, aqueous solutions of Cu⁺ are typically colourless.
- Therefore, E matches with U.
Matching the pairs:
A - S B - P C - Q D - T E - U
