Question
Question: Match the list: | | List-I | | List-II ...
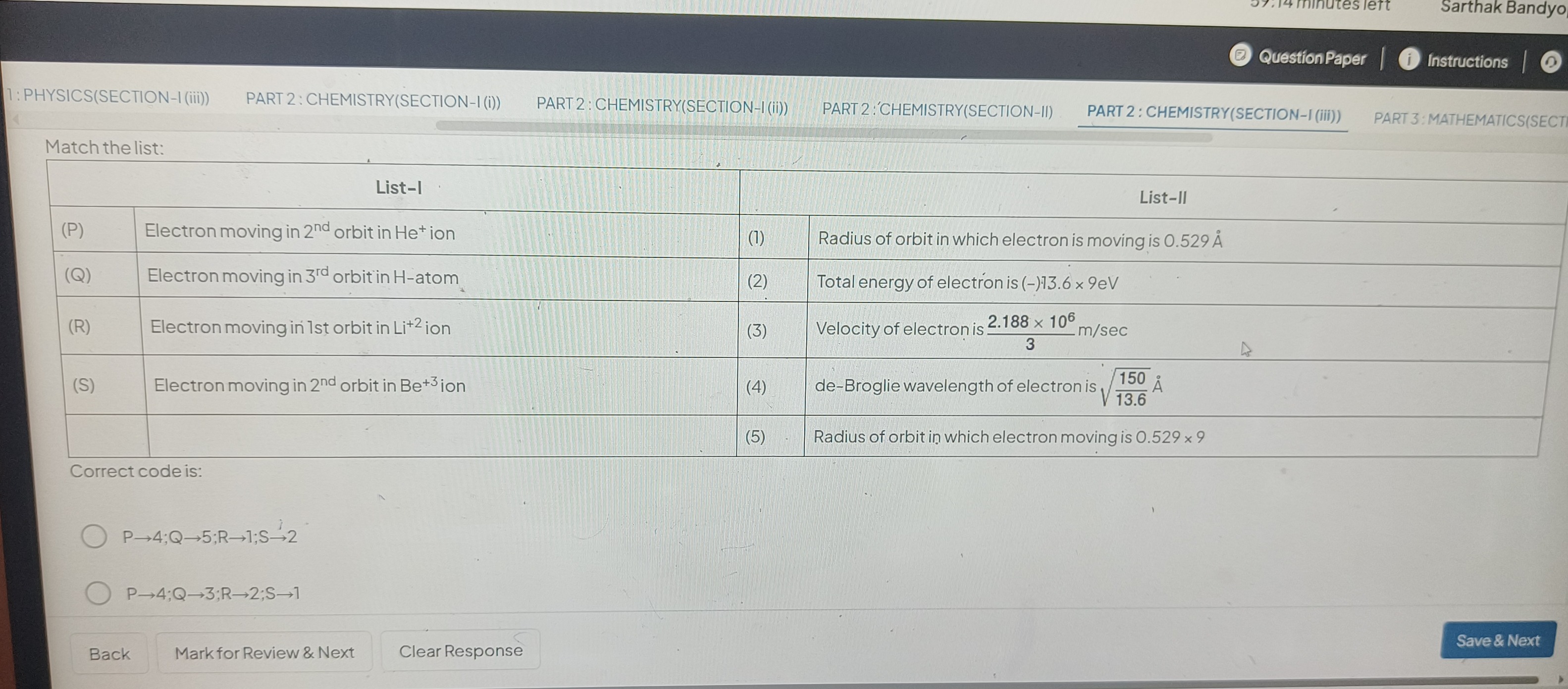
Match the list:
| List-I | List-II | ||
|---|---|---|---|
| (P) | Electron moving in 2nd orbit in He+ ion | (1) | Radius of orbit in which electron is moving is 0.529 Å |
| (Q) | Electron moving in 3rd orbit in H-atom | (2) | Total energy of electron is (-)13.6 x 9eV |
| (R) | Electron moving in 1st orbit in Li+2 ion | (3) | Velocity of electron is 32.188×106 m/sec |
| (S) | Electron moving in 2nd orbit in Be+3 ion | (4) | de-Broglie wavelength of electron is 13.6150A˚ |
| (5) | Radius of orbit in which electron moving is 0.529 x 9 |
A
(P) - (4), (Q) - (3), (R) - (2), (S) - (1)
B
(P) - (4), (Q) - (5), (R) - (2), (S) - (1)
C
(P) - (4), (Q) - (3) and (5), (R) - (2), (S) - (1)
D
(P) - (1), (Q) - (3), (R) - (2), (S) - (4)
Answer
(P) - (4), (Q) - (3) and (5), (R) - (2), (S) - (1)
Explanation
Solution
The problem requires matching properties of electrons in different atomic orbits (List-I) with specific values (List-II) using Bohr model formulas.
Formulas for Bohr Model:
- Radius of the n-th orbit: rn=Zn2a0, where a0=0.529 Å.
- Velocity of the electron in the n-th orbit: vn=nv1Z, where v1=2.188×106 m/sec.
- Total energy of the electron in the n-th orbit: En=−n213.6Z2 eV.
- de-Broglie wavelength: λn=n2πrn=Z2πna0.
Analysis of List-I items:
(P) Electron moving in 2nd orbit in He+ ion
- Species: He+ (Z=2)
- Orbit: n=2
- de-Broglie Wavelength: λ2=22π×2×0.529 Å =2π×0.529 Å ≈3.324 Å. Option (4) evaluation: 13.6150 Å ≈11.029 Å ≈3.321 Å. Thus, (P) matches (4).
(Q) Electron moving in 3rd orbit in H-atom
- Species: H-atom (Z=1)
- Orbit: n=3
- Radius: r3=132a0=9a0=9×0.529 Å. This matches option (5).
- Velocity: v3=3v1×1=32.188×106 m/sec. This matches option (3). Thus, (Q) matches both (3) and (5).
(R) Electron moving in 1st orbit in Li+2 ion
- Species: Li+2 (Z=3)
- Orbit: n=1
- Total Energy: E1=−1213.6×32=−13.6×9 eV. This matches option (2). Thus, (R) matches (2).
(S) Electron moving in 2nd orbit in Be+3 ion
- Species: Be+3 (Z=4)
- Orbit: n=2
- Radius: r2=422a0=44a0=a0=0.529 Å. This matches option (1). Thus, (S) matches (1).
Summary of Matches:
- (P) - (4)
- (Q) - (3)
- (Q) - (5)
- (R) - (2)
- (S) - (1)
The correct matches are: (P) - (4), (Q) - (3), (Q) - (5), (R) - (2), (S) - (1).
