Question
Question: Match the List-l with List-II. | | List-I (Reactions) | | List-...
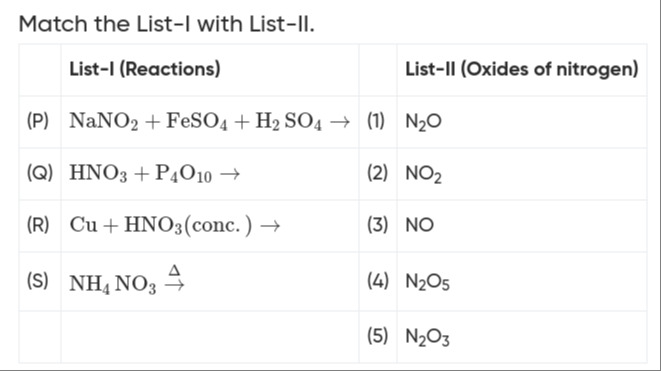
Match the List-l with List-II.
| List-I (Reactions) | List-II (Oxides of nitrogen) | ||
|---|---|---|---|
| (P) | NaNO2+FeSO4+H2SO4→ | (1) | N2O |
| (Q) | HNO3+P4O10→ | (2) | NO2 |
| (R) | Cu+HNO3(conc.)→ | (3) | NO |
| (S) | NH4NO3→Δ | (4) | N2O5 |
| (5) | N2O3 |
P - 1, Q - 2, R - 3, S - 4
P - 3, Q - 4, R - 2, S - 1
P - 5, Q - 4, R - 3, S - 2
P - 2, Q - 3, R - 4, S - 5
P-3, Q-4, R-2, S-1
Solution
The solution involves identifying the product oxides of nitrogen for each given reaction.
(P) NaNO2+FeSO4+H2SO4→
This reaction is part of the brown ring test. In an acidic medium, ferrous ions (Fe2+) reduce nitrite ions (NO2−) to nitric oxide (NO).
The ionic reaction is: NO2−+Fe2++2H+→NO+Fe3++H2O
Thus, the oxide of nitrogen produced is NO.
(P) matches with (3) NO.
(Q) HNO3+P4O10→
Phosphorus pentoxide (P4O10) is a powerful dehydrating agent. It dehydrates nitric acid (HNO3) to form dinitrogen pentoxide (N2O5).
The reaction is: 4HNO3+P4O10→2N2O5+4HPO3
Thus, the oxide of nitrogen produced is N2O5.
(Q) matches with (4) N2O5.
(R) Cu+HNO3(conc.)→
Copper reacts with concentrated nitric acid. Concentrated nitric acid acts as a strong oxidizing agent and is reduced to nitrogen dioxide (NO2).
The reaction is: Cu+4HNO3(conc.)→Cu(NO3)2+2NO2+2H2O
Thus, the oxide of nitrogen produced is NO2.
(R) matches with (2) NO2.
(S) NH4NO3→Δ
Ammonium nitrate (NH4NO3) decomposes on heating to produce nitrous oxide (N2O) and water. This is a redox reaction where nitrogen in the ammonium ion (oxidation state -3) is oxidized and nitrogen in the nitrate ion (oxidation state +5) is reduced.
The reaction is: NH4NO3→ΔN2O+2H2O
Thus, the oxide of nitrogen produced is N2O.
(S) matches with (1) N2O.
Final Matching:
(P) - (3)
(Q) - (4)
(R) - (2)
(S) - (1)
Explanation of the solution:
- (P) NaNO2+FeSO4+H2SO4→NO: Nitrite is reduced by Fe2+ in acidic medium to NO.
- (Q) HNO3+P4O10→N2O5: P4O10 dehydrates HNO3 to form N2O5.
- (R) Cu+HNO3(conc.)→NO2: Concentrated HNO3 oxidizes Cu and is itself reduced to NO2.
- (S) NH4NO3→ΔN2O: Thermal decomposition of ammonium nitrate yields N2O.
