Question
Question: Match the List-I with List-II. | List-I | List-II | | :----------------: | ...
Match the List-I with List-II.
| List-I | List-II |
|---|---|
| A. ICI⁻ | P. Linear |
| B. BrF₂⁺ | Q. Pyramidal |
| C. ClF₄⁻ | R. Tetrahedral |
| D. AlCl₄⁻ | S. Square planar |
| T. Angular |
Match the List-I with List-II.
| List-I | List-II |
|---|---|
| A. XeO₃ | P. Planar triangular |
| B. XeOF₄ | Q. T-shape |
| C. BO₃³⁻ | R. Trigonal pyramidal |
| D. ClF₃ | S. Square pyramidal |
| E. I₃⁻ (aq) | T. Linear |
| U. Bent |
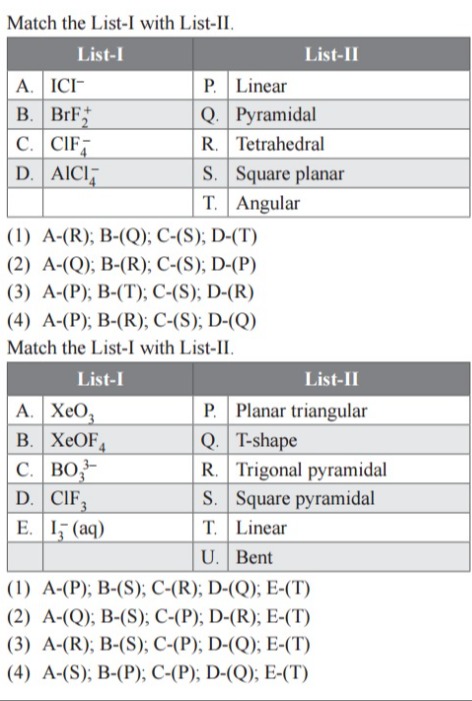
A-(R); B-(Q); C-(S); D-(T)
A-(Q); B-(R); C-(S); D-(P)
A-(P); B-(T); C-(S); D-(R)
A-(P); B-(R); C-(S); D-(Q)
3
Solution
The geometry of molecules and ions can be determined using the VSEPR (Valence Shell Electron Pair Repulsion) theory. This theory predicts the geometry based on the number of electron domains (bonding pairs and lone pairs) around the central atom, which repel each other and try to stay as far apart as possible.
Formulas used for VSEPR prediction:
- Steric Number (SN) = (Number of valence electrons on central atom + Number of monovalent atoms attached + |Negative charge| - Positive charge) / 2
- Number of Bonding Pairs (BP) = Number of atoms bonded to the central atom (count only sigma bonds for VSEPR geometry)
- Number of Lone Pairs (LP) = SN - BP
Based on SN, BP, and LP, the electron geometry and molecular geometry are determined.
Matching for the first question:
A. ICl₂⁻
- Central atom: I (7 valence electrons)
- Monovalent atoms: 2 Cl
- Charge: -1
- SN = (7 + 2 + 1) / 2 = 5
- BP = 2 (I-Cl bonds)
- LP = 5 - 2 = 3
- Electron geometry: Trigonal bipyramidal (SN=5)
- Molecular geometry (2 BP, 3 LP): The 3 lone pairs occupy equatorial positions, leading to a Linear shape.
- Match: P. Linear
B. BrF₂⁺
- Central atom: Br (7 valence electrons)
- Monovalent atoms: 2 F
- Charge: +1
- SN = (7 + 2 - 1) / 2 = 4
- BP = 2 (Br-F bonds)
- LP = 4 - 2 = 2
- Electron geometry: Tetrahedral (SN=4)
- Molecular geometry (2 BP, 2 LP): The two lone pairs repel the bonding pairs, resulting in an Angular (Bent) shape.
- Match: T. Angular
C. ClF₄⁻
- Central atom: Cl (7 valence electrons)
- Monovalent atoms: 4 F
- Charge: -1
- SN = (7 + 4 + 1) / 2 = 6
- BP = 4 (Cl-F bonds)
- LP = 6 - 4 = 2
- Electron geometry: Octahedral (SN=6)
- Molecular geometry (4 BP, 2 LP): The two lone pairs occupy opposite positions (180° apart), resulting in a Square planar shape.
- Match: S. Square planar
D. AlCl₄⁻
- Central atom: Al (3 valence electrons)
- Monovalent atoms: 4 Cl
- Charge: -1
- SN = (3 + 4 + 1) / 2 = 4
- BP = 4 (Al-Cl bonds)
- LP = 4 - 4 = 0
- Electron geometry: Tetrahedral (SN=4)
- Molecular geometry (4 BP, 0 LP): With no lone pairs, the molecular geometry is the same as the electron geometry, which is Tetrahedral.
- Match: R. Tetrahedral
Summary for Question 1: A - P B - T C - S D - R This corresponds to option (3).
Matching for the second question:
A. XeO₃
- Central atom: Xe (8 valence electrons)
- Bonded atoms: 3 O (form double bonds, so 3 sigma bonds)
- Electrons used in sigma bonds = 3 × 2 = 6
- Remaining valence electrons = 8 - 6 = 2 electrons = 1 lone pair.
- SN = Number of sigma bonds + Number of lone pairs = 3 + 1 = 4
- BP = 3
- LP = 1
- Electron geometry: Tetrahedral (SN=4)
- Molecular geometry (3 BP, 1 LP): Trigonal pyramidal.
- Match: R. Trigonal pyramidal
B. XeOF₄
- Central atom: Xe (8 valence electrons)
- Bonded atoms: 1 O (double bond, 1 sigma) + 4 F (single bonds, 4 sigma)
- Electrons used in sigma bonds = (1 × 2) + (4 × 2) = 10.
- Remaining valence electrons = 8 - 10 = -2. (This indicates expansion of octet). A more direct method for SN when oxygen is present: SN = (Valence electrons of central atom + Number of monovalent atoms) / 2 SN = (8 + 4) / 2 = 6
- BP = 5 (1 for O, 4 for F)
- LP = 6 - 5 = 1
- Electron geometry: Octahedral (SN=6)
- Molecular geometry (5 BP, 1 LP): Square pyramidal.
- Match: S. Square pyramidal
C. BO₃³⁻
- Central atom: B (3 valence electrons)
- Bonded atoms: 3 O (form 3 sigma bonds, due to resonance all are equivalent)
- Electrons used in sigma bonds = 3 × 2 = 6.
- Remaining valence electrons = 3 - 6 = -3. (Boron does not expand octet, this indicates formal charge distribution). For BO₃³⁻, boron forms 3 sigma bonds and has no lone pairs.
- SN = 3 (BP) + 0 (LP) = 3
- Electron geometry: Trigonal planar (SN=3)
- Molecular geometry (3 BP, 0 LP): Planar triangular (Trigonal planar).
- Match: P. Planar triangular
D. ClF₃
- Central atom: Cl (7 valence electrons)
- Monovalent atoms: 3 F
- Charge: 0
- SN = (7 + 3 + 0) / 2 = 5
- BP = 3 (Cl-F bonds)
- LP = 5 - 3 = 2
- Electron geometry: Trigonal bipyramidal (SN=5)
- Molecular geometry (3 BP, 2 LP): The two lone pairs occupy equatorial positions, leading to a T-shape geometry.
- Match: Q. T-shape
E. I₃⁻ (aq)
- Central atom: I (7 valence electrons)
- Monovalent atoms: 2 I (terminal I atoms)
- Charge: -1
- SN = (7 + 2 + 1) / 2 = 5
- BP = 2 (I-I bonds)
- LP = 5 - 2 = 3
- Electron geometry: Trigonal bipyramidal (SN=5)
- Molecular geometry (2 BP, 3 LP): The 3 lone pairs occupy equatorial positions, leading to a Linear shape.
- Match: T. Linear
Summary for Question 2: A - R B - S C - P D - Q E - T This corresponds to option (3).
Both questions have option (3) as the correct answer.
The final answer is 3
Explanation of the solution:
Question 1:
- ICl₂⁻: Central I, SN=5 (2 bonding pairs, 3 lone pairs) ⟹ Linear.
- BrF₂⁺: Central Br, SN=4 (2 bonding pairs, 2 lone pairs) ⟹ Angular (Bent).
- ClF₄⁻: Central Cl, SN=6 (4 bonding pairs, 2 lone pairs) ⟹ Square planar.
- AlCl₄⁻: Central Al, SN=4 (4 bonding pairs, 0 lone pairs) ⟹ Tetrahedral.
Question 2:
- XeO₃: Central Xe, SN=4 (3 bonding pairs, 1 lone pair) ⟹ Trigonal pyramidal.
- XeOF₄: Central Xe, SN=6 (5 bonding pairs, 1 lone pair) ⟹ Square pyramidal.
- BO₃³⁻: Central B, SN=3 (3 bonding pairs, 0 lone pairs) ⟹ Planar triangular.
- ClF₃: Central Cl, SN=5 (3 bonding pairs, 2 lone pairs) ⟹ T-shape.
- I₃⁻: Central I, SN=5 (2 bonding pairs, 3 lone pairs) ⟹ Linear.
Answer:
The correct option for both matching questions is (3).
