Question
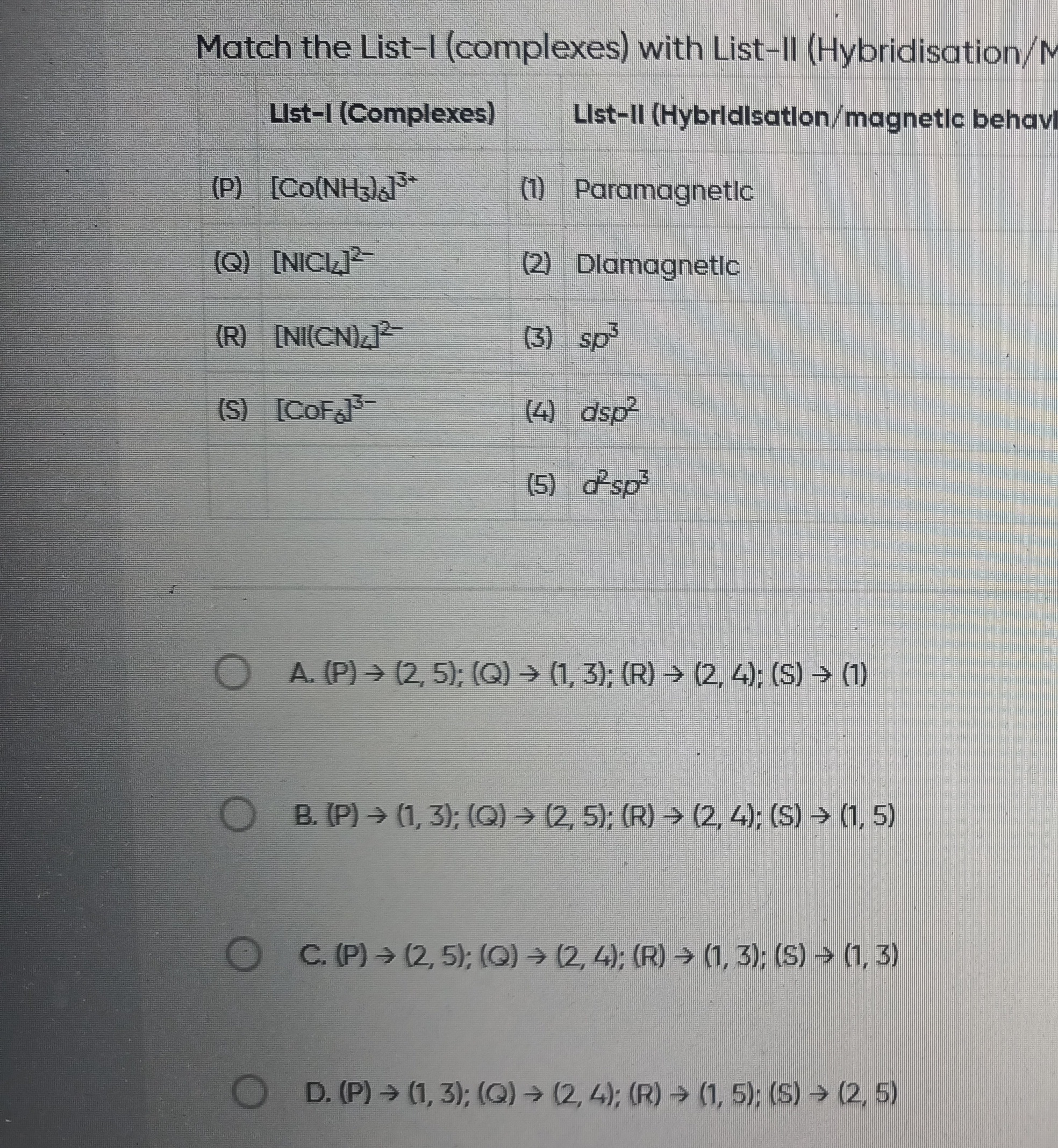
Question: Match the List-I (complexes) with List-II (Hybridisation/magnetic behavi | List-I (Complexes) | Lis...
Match the List-I (complexes) with List-II (Hybridisation/magnetic behavi
| List-I (Complexes) | List-II (Hybridisation/magnetic behavi |
|---|---|
| (P) [Co(NH3)6]3+ | (1) Paramagnetic |
| (Q) [NiCl4]2- | (2) Dlamagnetic |
| (R) [Ni(CN)4]2- | (3) sp³ |
| (S) [CoF6]3- | (4) dsp² |
| (5) d²sp³ |
A
(P) → (2, 5); (Q) → (1, 3); (R) → (2, 4); (S) → (1)
B
(P) → (1, 3); (Q) → (2, 5); (R) → (2, 4); (S) → (1,5)
C
(P) → (2, 5); (Q) → (2, 4); (R) → (1, 3); (S) → (1, 3)
D
(P) → (1, 3); (Q) → (2, 4); (R) → (1, 5); (S) → (2,5)
Answer
A. (P) → (2, 5); (Q) → (1, 3); (R) → (2, 4); (S) → (1)
Explanation
Solution
-
Oxidation States and d-electron Configuration:
- (P) [Co(NH₃)₆]³⁺: Co³⁺ (d⁶)
- (Q) [NiCl₄]²⁻: Ni²⁺ (d⁸)
- (R) [Ni(CN)₄]²⁻: Ni²⁺ (d⁸)
- (S) [CoF₆]³⁻: Co³⁺ (d⁶)
-
Ligand Strength, Hybridization, and Magnetic Behavior:
- (P) [Co(NH₃)₆]³⁺: NH₃ (strong ligand) → d²sp³ hybridization, diamagnetic (d⁶ pairs up). Matches (2) and (5).
- (Q) [NiCl₄]²⁻: Cl⁻ (weak ligand) → sp³ hybridization, paramagnetic (d⁸ has unpaired electrons). Matches (1) and (3).
- (R) [Ni(CN)₄]²⁻: CN⁻ (strong ligand) → dsp² hybridization, diamagnetic (d⁸ pairs up). Matches (2) and (4).
- (S) [CoF₆]³⁻: F⁻ (weak ligand) → sp³d² hybridization, paramagnetic (d⁶ has unpaired electrons). Matches (1).
-
Final Matches:
- (P) → (2, 5)
- (Q) → (1, 3)
- (R) → (2, 4)
- (S) → (1)
This corresponds to Option A.
