Question
Question: Match the following: **Natural Source**| **Acid** ---|--- 1\. Vinegar| A. Oxalic Acid 2\. ...
Match the following:
| Natural Source | Acid |
|---|---|
| 1. Vinegar | A. Oxalic Acid |
| 2. Orange | B. Acetic Acid |
| 3. Tamarind | C. Citric Acid |
| 4. Tomato | D. Tartaric acid |
A. 1-a, 2-b, 3-d, 4-c
B. 1-b, 2-c, 3-d, 4-a
C. 1-a, 2-c, 3-d, 4-a
D. 1-d, 2-a, 3-b, 4-c
Solution
The chemical formula and name of the acid present in the vinegar is CH3COOH ethanoic acid. The chemical formula and name of the acid present in the orange is C6H8O7 2−hydroxy propane−1,2,3−tricarboxylic acid. The chemical formula and name of the acid present in the tamarind is C4H6O6 2,3−Dihydroxy butanedioic acid. The chemical formula and name of the acid present in the tomato is C2H2O4 ethanedioic acid.
Complete answer:
A molecule that can donate a proton or accept electrons is known as acid.
The acid present in vinegar is acetic acid which is systematically known as ethanoic acid. It is a weak acid. The chemical formula of the acetic acid is CH3COOH.
The structure of acetic acid is as follows:
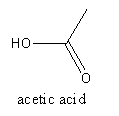
The acid present in orange is citric acid which is systematically known as 2− hydroxy propane−1,2,3−tricarboxylic acid. It is soluble in water. The molecular formula of the citric acid is C6H8O7.
The structure of citric acid is as follows:
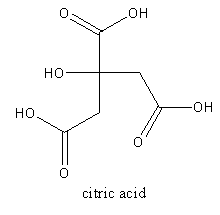
The acid present in tamarind is tartaric acid. It is an alpha-hydroxy carboxylic acid. The molecular formula of the tartaric acid is C4H6O6. The IUPAC name of tartaric acid is 2,3−Dihydroxy butanedioic acid.
The structure of tartaric acid is as follows:
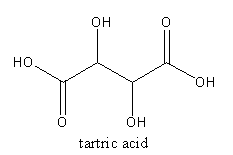
The acid present in tomato is oxalic acid. The molecular formula of the tartaric acid is C2H2O4. The systematic name of oxalic acid is ethanedioic acid.
The structure of oxalic acid is as follows:
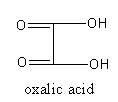
So, correct match of natural source and acid are as following:
| Natural Source | Acid |
|---|---|
| 1. Vinegar | B. Acetic Acid |
| 2. Orange | C. Citric Acid |
| 3. Tamarind | D. Tartaric Acid |
| 4. Tomato | A. Oxalic Acid |
**Therefore, option (B) 1-b, 2-c, 3-d, 4-a, is correct.
Note:** All acids have two carboxylic groups. The substance which has acids has a sour test. The pH of the liquid of these substances will be less than seven. Liquid of these substances can convert blue litmus into red which confirms the acidic nature of these substances. The main acid present in orange is ascorbic acid or vitamin C having molecular formula C6H8O6. Ascorbic acid or vitamin C cannot be produced by the body and cannot be stored. It is obtained from the diet. It is necessary for the recovery of the tissues. Fruit and vegetables are rich sources of ascorbic acid.
