Question
Question: Match the following 1\. Natural sources a. Acid 2\. Sour milk (Curd) b. Lactic acid 3\. Lemon ...
Match the following
1. Natural sources a. Acid
2. Sour milk (Curd) b. Lactic acid
3. Lemon c. citric acid
4. Ant sitting d. Methanoic acid
A. 1−a, 2−b, 3−c
B. 1−a, 2−c, 3−b
C. 1−c, 2−b, 3−a
D. 1−b, 2−a, 3−c
Solution
We know that in a chemical reaction, chemical equilibrium is the state where the rates of forward reaction and the reverse reaction are the same. The result is that the concentration of the reactants and the products remains unchanged. The rate constant of the forward reaction divided by the rate constant of the reverse reaction gives the equilibrium constant of a reaction. We can calculate the value of equilibrium constant by multiplying the concentration of the products and dividing it by the concentration of reactants. We can calculate the concentration of the reactants and products, their moles and volume.
Complete step by step answer:
Milk is proteins, lactose, fat, water, and minerals. Pasteurized milk canned or dry are acid-forming food. Its pH level is below neutral at about 6.7 to 6.9. This is due to lactic acid.
Now we are going to discuss the Lactic acid chemical composition CH3CH(OH)COOH. Lactobacillus bacteria are fermentation milk (sour milk) into curd lactic acid. There is a sour flavor of sourdough bread. Racemic mixture lactic acid is synthesized by reacting acetaldehyde with hydrogen cyanide and treated with water the resultant lacto nitrile. Now we can draw a structure,
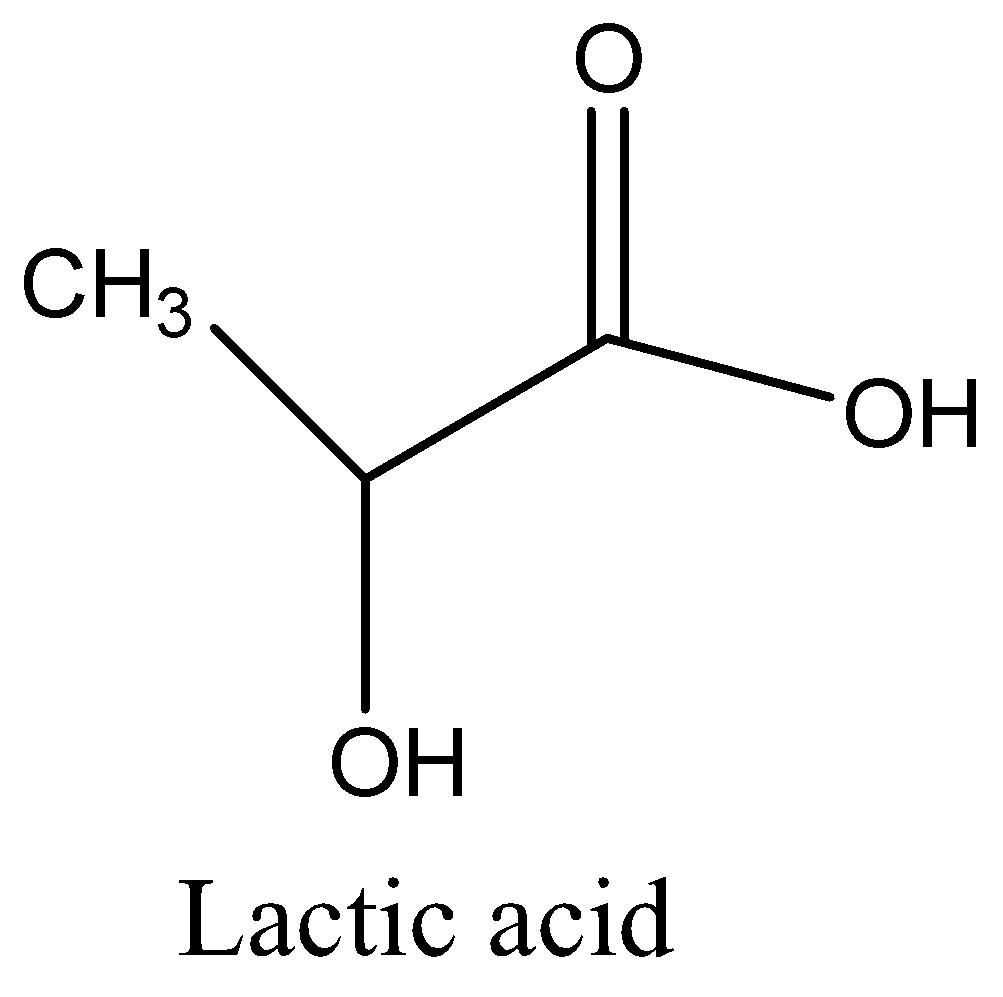
Now we are going to discuss about the Citric acid is consist of a three carboxylic acid groups(COOH) , which has alpha-hydroxy acid with a three carbon skeleton show tribasic acid, and one hydroxyl group (OH) is a weak acid, shows pH level between 3 to 6.Molecular formula is C6H8O7main source of citric acid is lemon and orange. Now we can draw a structure,
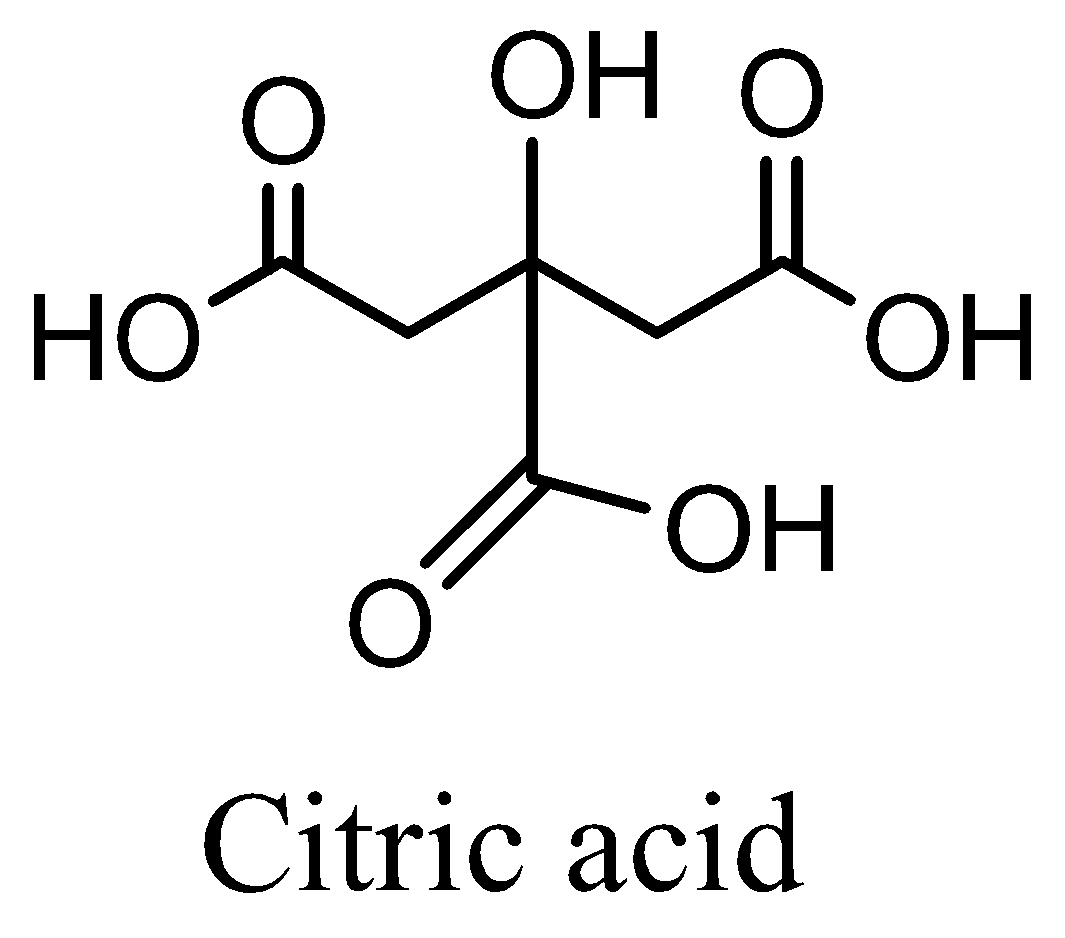
Now we are going to discuss whether the Methonic acid is well known as formic acid. It is carboxylic acid having the chemical formulae HCOOH (weak acid) that occurs in natural ants. Ants release the methonic acid for defense purposes and attack. Concentrated formic acid is corrosive and can cause burns to any part of the body it comes into contact with. Ingestion of formic acid can result in burns to the throat, mouth and stomach. Now we can draw the
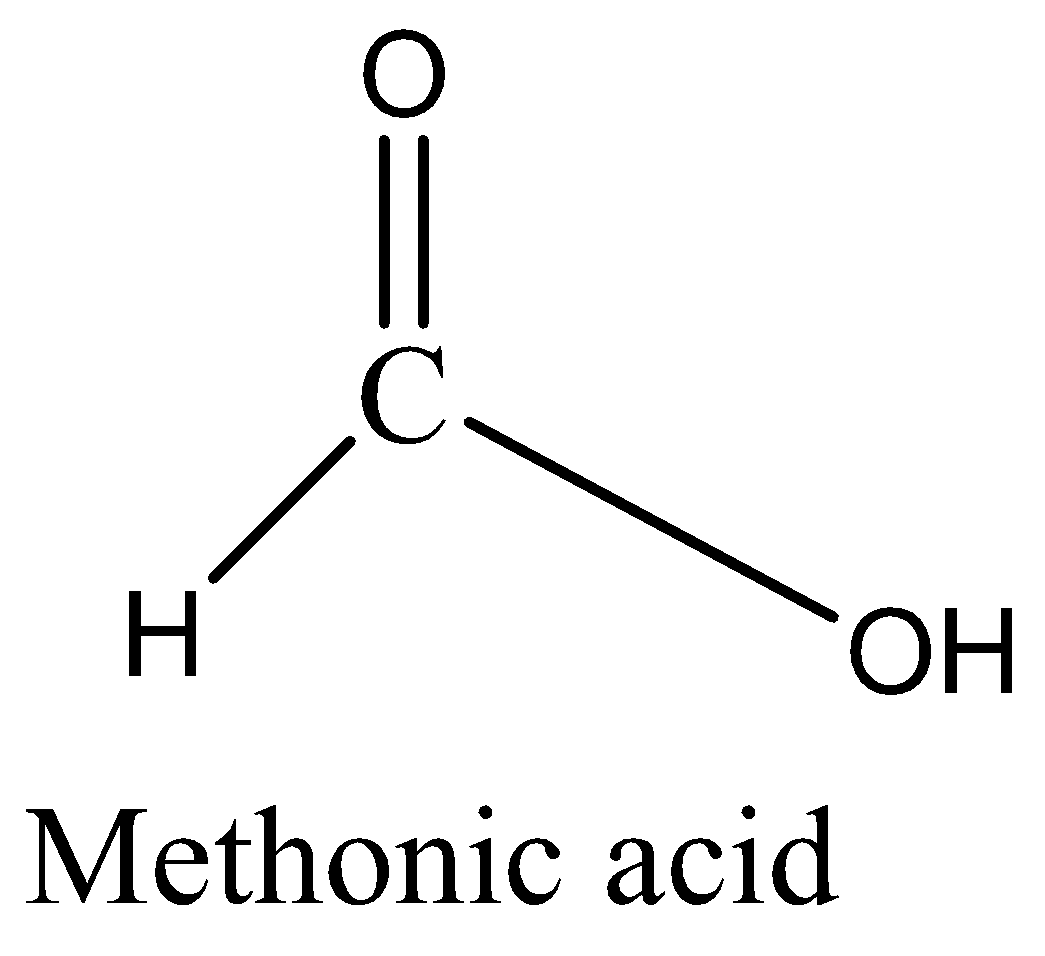
When methanol reacts with carbon monoxide gives methyl formate as a strong base further its hydrolysis to get methonic acid.
CH3OH + CO → HCO2CH3
HCO2CH3 + H2O → HCOOH + CH3OH
So, the correct answer is Option A .
Note:
We have to mind that the uses,
Uses of lactic acid are liquid cleaner and sour of milk.
Uses of citric acid are edible acid and chelating agent.
Uses of methonic acid are preservative and antibacterial agents.
