Question
Question: Match the column: | | Column-I (Reaction) ...
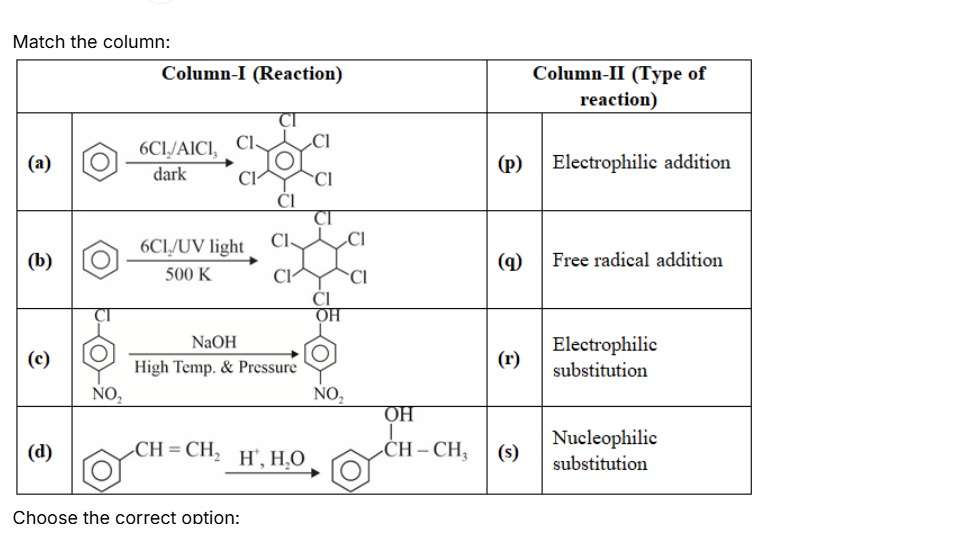
Match the column:
| Column-I (Reaction) | Column-II (Type of reaction) | ||
|---|---|---|---|
| (a) | (p) | Electrophilic addition | |
| (b) | (q) | Free radical addition | |
| (c) | (r) | Electrophilic substitution | |
| (d) | (s) | Nucleophilic substitution |
Choose the correct option:
a - r
b - q
c - s
d - p
a-r, b-q, c-s, d-p
Solution
The problem requires us to match each reaction in Column-I with its corresponding type in Column-II. We will analyze each reaction:
(a) Reaction: Benzene reacts with chlorine in the presence of a Lewis acid (AlCl₃). This is a typical electrophilic aromatic substitution (EAS) reaction. The AlCl₃ helps generate the electrophile Cl⁺, which then attacks the benzene ring, replacing hydrogen atoms with chlorine atoms. Therefore, reaction (a) is an Electrophilic substitution reaction.
(b) Reaction: Benzene reacts with chlorine under UV light and high temperature. These conditions are characteristic of free radical reactions. The UV light initiates the homolytic cleavage of Cl₂ to form chlorine free radicals (Cl•). These free radicals add across the double bonds of the benzene ring, leading to the loss of aromaticity and the formation of a saturated cyclic compound. Therefore, reaction (b) is a Free radical addition reaction.
(c) Reaction: This reaction involves the replacement of a chlorine atom attached to an aromatic ring by a hydroxyl group (-OH) from NaOH. This is a nucleophilic aromatic substitution (SNAr) reaction. The strong electron-withdrawing nitro group (-NO₂) at the para position activates the aryl halide towards nucleophilic attack by stabilizing the intermediate carbanion (Meisenheimer complex). Therefore, reaction (c) is a Nucleophilic substitution reaction.
(d) Reaction: This is the acid-catalyzed hydration of an alkene (styrene). Alkenes typically undergo electrophilic addition reactions. The H⁺ acts as an electrophile, attacking the double bond to form a carbocation (specifically, a more stable secondary benzylic carbocation according to Markovnikov's rule). This carbocation is then attacked by water (a nucleophile), followed by deprotonation to yield the alcohol. Therefore, reaction (d) is an Electrophilic addition reaction.
Summary of matches: (a) → (r) (b) → (q) (c) → (s) (d) → (p)
