Question
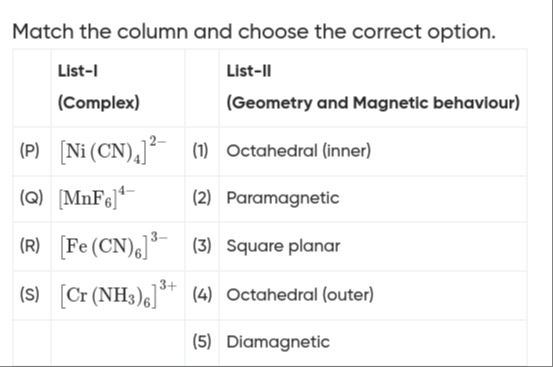
Question: Match the column and choose the correct option. | | List-I (Complex) ...
Match the column and choose the correct option.
| List-I (Complex) | List-II (Geometry and Magnetic behaviour) | ||
|---|---|---|---|
| (P) | [Ni(CN)4]2− | (1) | Octahedral (inner) |
| (Q) | [MnF6]4− | (2) | Paramagnetic |
| (R) | [Fe(CN)6]3− | (3) | Square planar |
| (S) | [Cr(NH3)6]3+ | (4) | Octahedral (outer) |
| (5) | Diamagnetic |
(P)-3,5; (Q)-4,2; (R)-1,2; (S)-1,2
Solution
-
[Ni(CN)4]2−: Ni is +2 (3d8). CN− is a strong field ligand. This leads to dsp2 hybridization, resulting in a square planar geometry. The 3d8 configuration with a strong field ligand results in all electrons being paired, making it diamagnetic. (P) matches with (3) and (5).
-
[MnF6]4−: Mn is +2 (3d5). F− is a weak field ligand. This leads to sp3d2 hybridization, resulting in an outer octahedral geometry. The 3d5 configuration with a weak field ligand has 5 unpaired electrons, making it paramagnetic. (Q) matches with (4) and (2).
-
[Fe(CN)6]3−: Fe is +3 (3d5). CN− is a strong field ligand. This leads to d2sp3 hybridization, resulting in an inner octahedral geometry. The 3d5 configuration with a strong field ligand has 1 unpaired electron, making it paramagnetic. (R) matches with (1) and (2).
-
[Cr(NH3)6]3+: Cr is +3 (3d3). NH3 is a moderately strong field ligand. This leads to d2sp3 hybridization, resulting in an inner octahedral geometry. The 3d3 configuration has 3 unpaired electrons, making it paramagnetic. (S) matches with (1) and (2).
