Question
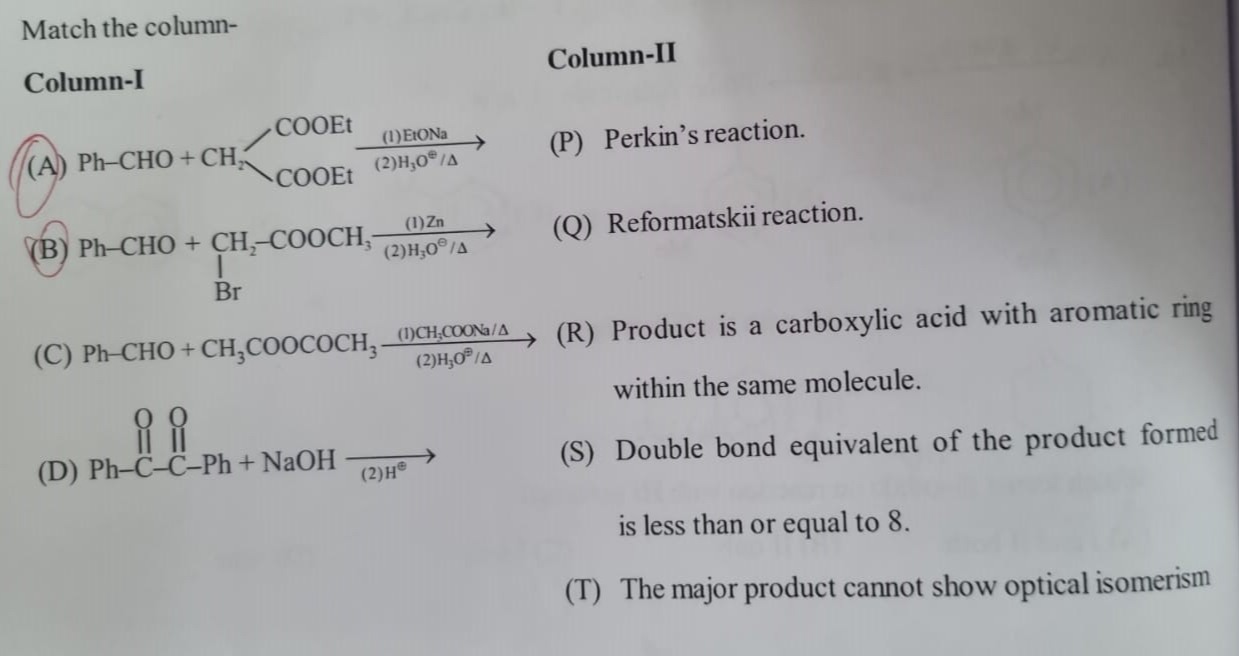
Question: Match the column- Column-I | Column-II ---|--- (A) Ph-CHO + $CH_2 \begin{matrix} \text{COOEt} \\ \t...
Match the column-
| Column-I | Column-II |
|---|---|
| (A) Ph-CHO + CH2COOEtCOOEt(1)EtONa(2)H3O⊕/Δ | (P) Perkin's reaction. |
| (B) Ph-CHO + CH2-COOCH3IBr(1)Zn(2)H3O⊕/Δ | (Q) Reformatskii reaction. |
| (C) Ph-CHO + CH3COOCOCH3(1)CH3COONa/Δ(2)H3O⊕/Δ | (R) Product is a carboxylic acid with aromatic ring within the same molecule. |
| (D) Ph-O∥C-O∥C-Ph + NaOH (2)H⊕ | (S) Double bond equivalent of the product formed is less than or equal to 8. |
| (T) The major product cannot show optical isomerism |
(A) - (R), (S), (T)
(B) - (Q), (R), (S), (T)
(C) - (P), (R), (S), (T)
(D) - (R), (T)
(A)-(R,S,T), (B)-(Q,R,S,T), (C)-(P,R,S,T), (D)-(R,T)
Solution
Let's analyze each reaction in Column-I and match it with the descriptions in Column-II.
(A) Ph-CHO + CH2COOEtCOOEt(1)EtONa(2)H3O⊕/Δ
This is a Knoevenagel condensation between benzaldehyde and diethyl malonate. The product, after hydrolysis and decarboxylation, is Cinnamic acid (Ph-CH=CH-COOH).
- (R) Product is a carboxylic acid with aromatic ring: Yes, Cinnamic acid.
- (S) Double bond equivalent (DBE) of Cinnamic acid (C9H8O2): DBE = C + (N/2) - (H/2) + 1 = 9 + 0 - (8/2) + 1 = 9 - 4 + 1 = 6. 6 ≤ 8. Yes.
- (T) The major product cannot show optical isomerism: Cinnamic acid has no chiral center and is achiral. Yes.
- (P) Perkin's reaction: No.
- (Q) Reformatskii reaction: No.
Matches for (A): (R), (S), (T).
(B) Ph-CHO + CH2-COOCH3IBr(1)Zn(2)H3O⊕/Δ
This is a Reformatskii reaction between benzaldehyde and methyl α-bromoacetate. The intermediate β-hydroxy ester undergoes hydrolysis and dehydration upon heating to form Cinnamic acid (Ph-CH=CH-COOH).
- (Q) Reformatskii reaction: Yes.
- (R) Product is a carboxylic acid with aromatic ring: Yes, Cinnamic acid.
- (S) DBE of Cinnamic acid is 6. 6 ≤ 8. Yes.
- (T) The major product cannot show optical isomerism: Cinnamic acid is achiral. Yes.
- (P) Perkin's reaction: No.
Matches for (B): (Q), (R), (S), (T).
(C) Ph-CHO + CH3COOCOCH3(1)CH3COONa/Δ(2)H3O⊕/Δ
This is a Perkin's reaction between benzaldehyde and acetic anhydride in the presence of sodium acetate. The product, after hydrolysis, is Cinnamic acid (Ph-CH=CH-COOH).
- (P) Perkin's reaction: Yes.
- (R) Product is a carboxylic acid with aromatic ring: Yes, Cinnamic acid.
- (S) DBE of Cinnamic acid is 6. 6 ≤ 8. Yes.
- (T) The major product cannot show optical isomerism: Cinnamic acid is achiral. Yes.
- (Q) Reformatskii reaction: No.
Matches for (C): (P), (R), (S), (T).
(D) Ph-O∥C-O∥C-Ph + NaOH (2)H⊕
This is a Benzilic acid rearrangement of benzil (1,2-diketone) in the presence of a strong base. The product is Benzilic acid (Ph2C(OH)COOH).
- (R) Product is a carboxylic acid with aromatic ring: Yes, Benzilic acid has two phenyl rings.
- (T) The major product cannot show optical isomerism: Benzilic acid (Ph2C(OH)COOH) has a carbon bonded to two identical phenyl groups, a hydroxyl group, and a carboxyl group. It is achiral. Yes.
- (S) DBE of Benzilic acid (C14H12O3): DBE = C + (N/2) - (H/2) + 1 = 14 + 0 - (12/2) + 1 = 14 - 6 + 1 = 9. 9 is not ≤ 8. No.
- (P) Perkin's reaction: No.
- (Q) Reformatskii reaction: No.
Matches for (D): (R), (T).
Final Matching:
(A) - (R), (S), (T) (B) - (Q), (R), (S), (T) (C) - (P), (R), (S), (T) (D) - (R), (T)
