Question
Question: Match List - I with List - Il and select the correct answer using the codes given below the lists: L...
Match List - I with List - Il and select the correct answer using the codes given below the lists: List-I (Compound)List-11 (of Central atom)
| List 1 | List 2 |
|---|---|
| [Ni(NH3)6]2+ | sp3 |
| [PtCl4]2− | sp3d2 |
| [Ni(CO)4] | dsp2 |
| [Co(ox)3]3− | d2sp3 |
(i).(A)- 2, (B)-1 (C) - 3.(D) -4
(ii). (A) -2 (B) -3,(C) 1. (D) -4
(iii) (A) - 4. (1) -1(C) -3, (D) – 2
(iv) (A) - 4. (D) -3 (C) -1 (D) - 2
Solution
Hint The concept of Valence Bond theory is to be used while solving this question. It is usually possible to predict the geometry of a complex from the knowledge of its magnetic behaviour on the basis of VBT.
Complete step by step solution:
In order to answer this question, we need to learn about the coordination compounds and the valence bond theory. According to valence bond theory, the metal atom or ion can use (n-1)d, ns, np. nd orbitals for hybridisation under the influence of ligands to yield a set of equivalent orbitals of definite geometry such as square planar tetrahedral, octahedral and so on These hybrid orbitals and ligand orbitals are allowed to overlap with each other that later can donate electron pair for bonding. For (n- 1)d orbitals used in hybridisation, there is formation of inner orbital complex, and for np or nd orbitals the outer orbital complex formation tan place. Generally inner orbital complexes are low spin complexes with lesser number of unpaired electrons while outer orbital complexes are generally high spin complexes with more numbers of unpaired electrons. The applications of VBT are:
Tetrahedral complex sp3: One ns orbital and three np orbitals are involved in hybridisation. For example, in [NiCl4]2− nickel ion has +2 oxidation state and has configuration of 3d64s0. Each Cl− donate a pair of electrons. The compound is paramagnetic since it contains two unpaired electrons. Cl−, being a weak ligand, cannot pair the unpaired electrons of Ni2+.
Square planar complex dsp2: One (n-1)d orbital, one ns orbital and two np orbitals (any two of np, np, and np.) are involved in hybridisation. For example, [Ni(CN)4]2− nickel ion has +2 oxidation state and has configuration of 3d64s0. CN−, being strong ligand, pairs up the unpaired electrons of Ni2+ ion.
Inner and outer orbital complex d2sp3andsp3d2: d2sp3 hybridisation involves two (n-1)d, one ns and three np orbitals; while sp3d2 hybridisation involves one ns, three np and two nd orbitals.
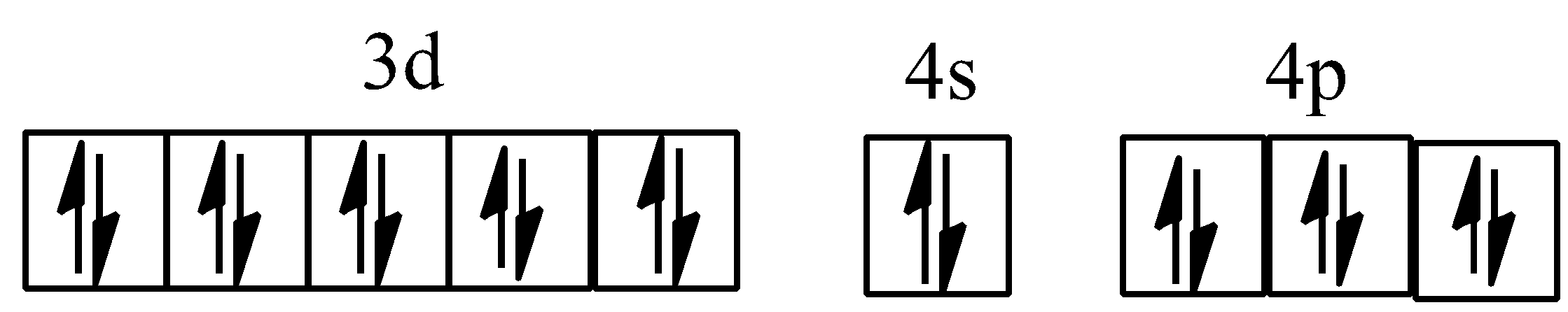
For example, in [Co(NH3)6]3+, cobalt ion has +3 oxidation state and has configuration of 3d64s0. NH3, here becomes stronger ligand due to +3 oxidation state of cobalt ion, paired up the unpaired electrons of Co3+. Complex is diamagnetic due to the absence of an unpaired electron. Since the inner d-orbital (3d) is used in hybridisation, [Co(NH3)6]3+is called an inner orbital or low spin or spin paired complex.
Hybridisation of [Ni(NH3)6]2+
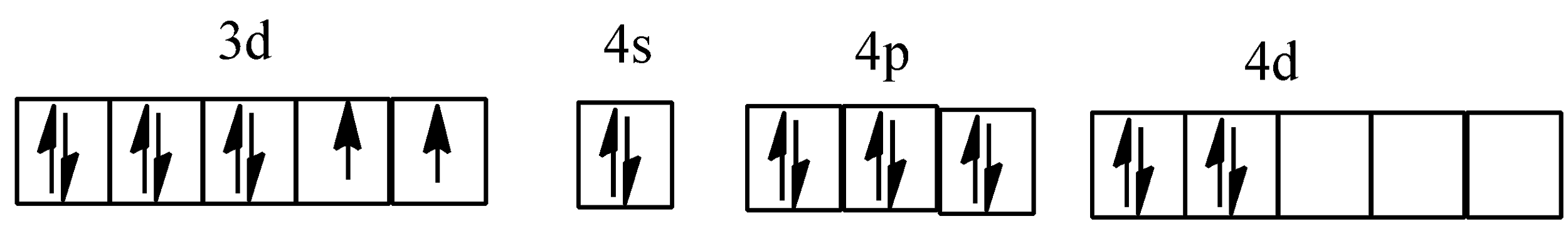
Hybridisation of [PtCl4]2−
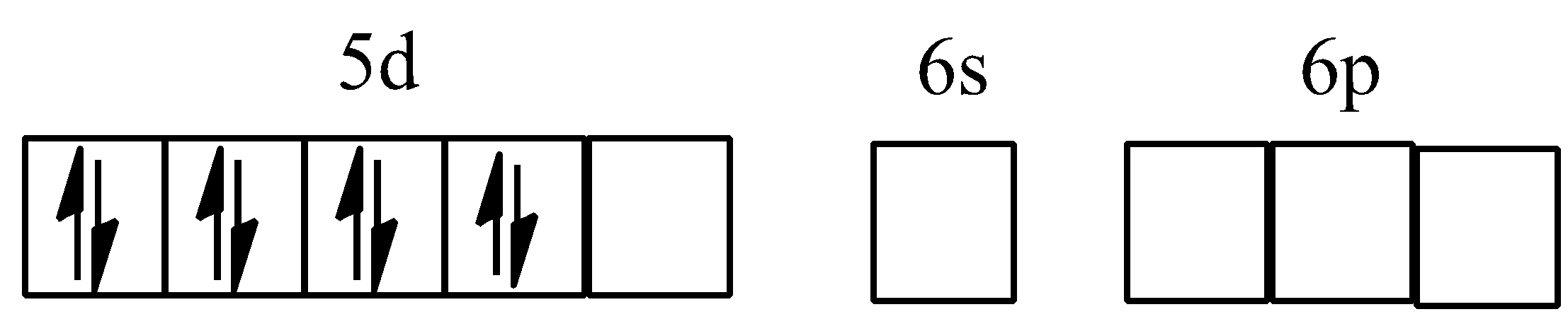
Hybridisation of [Ni(CO)4]
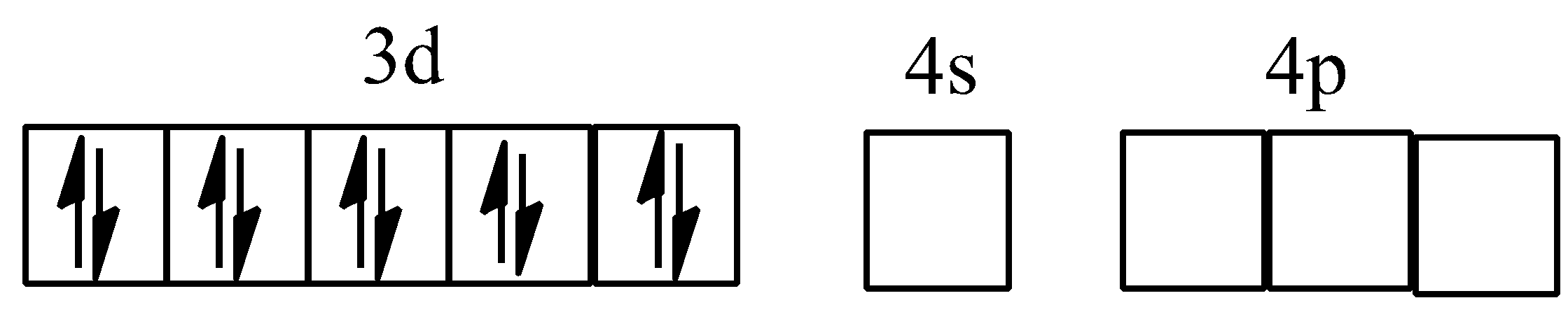
Hybridisation of [Co(ox)3]3−
The structures of the compounds are:
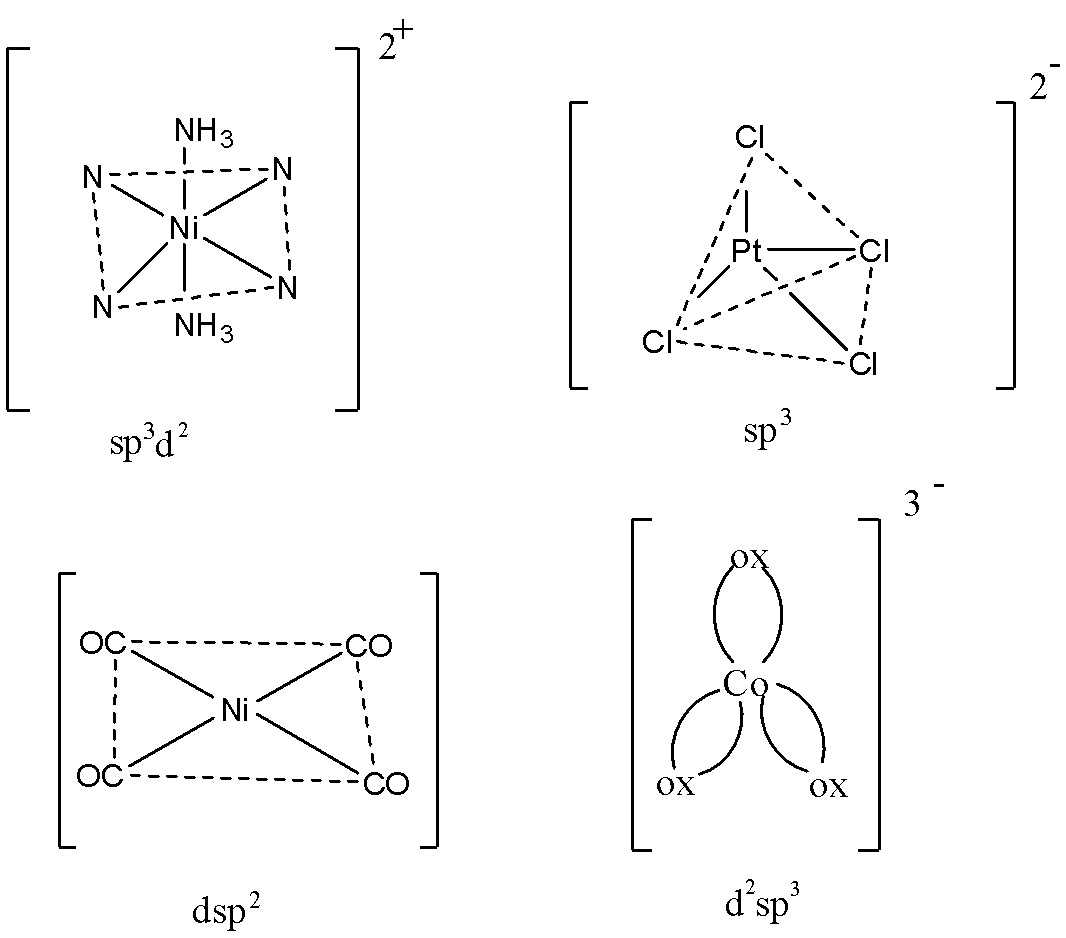
So, seeing the structures, we can say that the correct answer would be option A.
| Option A | (2) |
|---|---|
| Option B | (1) |
| Option C | (3) |
| Option D | (4) |
NOTE: [Ni(CO)4] has tetrahedral geometry, but it is diamagnetic, since Ni is in zero oxidation state and contains no unpaired electrons CO being strong ligand, pairs up the unpaired electrons of Ni(0) against Hund's rule of maximum multiplicity.
