Question
Question: Match LIST-I with LIST-II: | | LIST-I (Molecule) | | LIST-II (Shape) | | :---- | :-...
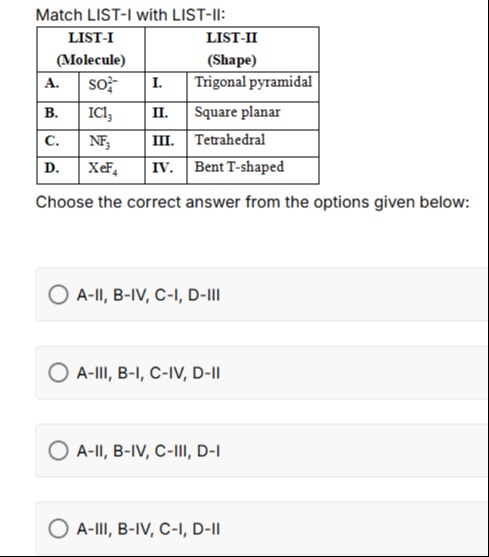
Match LIST-I with LIST-II:
| LIST-I (Molecule) | LIST-II (Shape) | ||
|---|---|---|---|
| A. | SO42− | I. | Trigonal pyramidal |
| B. | ICl3 | II. | Square planar |
| C. | NF3 | III. | Tetrahedral |
| D. | XeF4 | IV. | Bent T-shaped |
Choose the correct answer from the options given below:
A
A-II, B-IV, C-I, D-III
B
A-III, B-I, C-IV, D-II
C
A-II, B-IV, C-III, D-I
D
A-III, B-IV, C-I, D-II
Answer
A-III, B-IV, C-I, D-II
Explanation
Solution
Solution:
- SO42−: Has 4 bonding pairs and no lone pairs → Tetrahedral geometry → Option III.
- ICl3: Iodine has 5 electron groups (3 bonds + 2 lone pairs) → T-shaped molecular geometry → Option IV ("Bent T-shaped").
- NF3: 4 electron groups (3 bonds + 1 lone pair) → Trigonal pyramidal → Option I.
- XeF4: 6 electron groups (4 bonds + 2 lone pairs arranged opposite) → Square planar → Option II.
Thus, the matching is: A-III, B-IV, C-I, D-II.
