Question
Question: Match list-I and list-II **List I**| **List II** ---|--- A. Hypophosphoric acid (\[{{\text{H...
Match list-I and list-II
| List I | List II |
|---|---|
| A. Hypophosphoric acid (H4P2O6) | 1. Non-reducing |
| B. Isohypophosphoric acid (H4P2O6) | 2. P in +4 state |
| C. Pyrophosphorous acid (H4P2O5) | 3. P in +3 state |
| D. Pyrophosphoric acid (H4P2O7) | 4. P-P bond |
| 5. P-O-P bond |
a. A-1,2,4; B-3,5; C-3,5; D-1,5
b. A-1,2; B-3,4; C-3,5; D-5
c. A-2; B-3,5; C-3; D-1,4
d. A-4; B-1,3,5; C-4,5; D-2,5
Solution
Draw the structure of all acids. Acid which does not contain P - H bond is known as non-reducing acid. Use the oxidation number rules to determine the oxidation state of P atom.
Complete answer:
A. The structure of hypophosphoric acid (H4P2O6) is as follows:
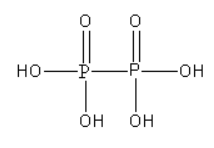
From the structure of hypophosphoric acid, we can say that there is a P-P bond present in it. However, there is no P-O-P bond in it.
As hypophosphoric acid does not contain P - H bond it is known as non-reducing.
In hypophosphoric acid, both P atoms are surrounded by the same groups so we can determine the oxidation state P atom using the oxidation number rules.
According to the oxidation number rule, the oxidation state of oxygen is -2 and the oxidation state of hydrogen is +1.
In hypophosphoric acid, there are two P atoms, four H atoms and six O atoms.
(Number of P atoms) (Oxidation state of P) + (Number of O atoms) (Oxidation state of O) + (Number of H atom)(oxidation state of H atom)= 0
2 (Oxidation state of P) + 6(-2)+ 4(+1) = 0
Oxidation state of P = +4
Thus, Hypophosphoric acid (H4P2O6) matches with 1, 2, 4 from list II.
Hence, for hypophosphoric acid A-1, 2, 4 is the correct answer.
B. The structure of Isohypophosphoric acid (H4P2O6) is as follows:
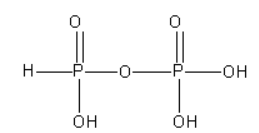
From the structure of isohypophosphoric acid we can say that there is a P-O-P bond present in it. However, there is no P-P bond in it.
As isohypophosphoric acid contains the P - H bond it is known as reducing.
Both P atoms are surrounded by different groups so the oxidation state of both P atoms is different.
The oxidation state of P atom bonded to H atom is +3 while the oxidation state of another P atom is +5.
Thus, isohypophosphoric acid (H4P2O6) matches with 3,5 from list II.
Hence, for isohypophosphoric acid B-3,5 is the correct answer.
C. The structure of pyrophosphorous acid (H4P2O5) is as follows:
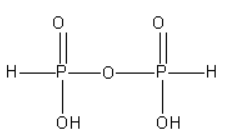
From the structure of pyrophosphorous acid, we can say that there is a P-O-P bond present in it. However, there is no P-P bond in it.
As of pyrophosphorous acid contains the P - H bond it is known as reducing.
In pyrophosphorous acid, both P atoms are surrounded by the same groups. The oxidation state of both P atoms is +3.
Thus, of pyrophosphorous acid (H4P2O5) matches with 3,5 from list II.
Hence, for pyrophosphorous acid C-3,5 is the correct answer.
D. The structure of pyrophosphoric acid (H4P2O7) is as follows:
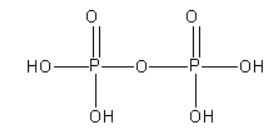
From the structure of pyrophosphoric acid, we can say that there is a P-O-P bond present in it. However, there is no P-P bond in it.
As pyrophosphoric acid does not contain P - H bond it is known as non-reducing.
In pyrophosphoric acid, both P atoms are surrounded by the same groups. The oxidation state of both P atoms is +5.
Thus, of pyrophosphoric acid (H4P2O7) matches with 1,5 from list II.
Hence, for pyrophosphoric acid D-1,5 is the correct answer.
**Thus, correct option is (a) A-1, 2, 4; B-3, 5; C-3, 5; D-1, 5
Note:**
Acids are proton donor species. Proton bonded to the oxygen atom is known as an acidic proton. Proton bonded directly to the central atom is known as reducing proton.
