Question
Question: Mark the correct order of decreasing acid strength of the following compounds. (I) (II) (III) (I...
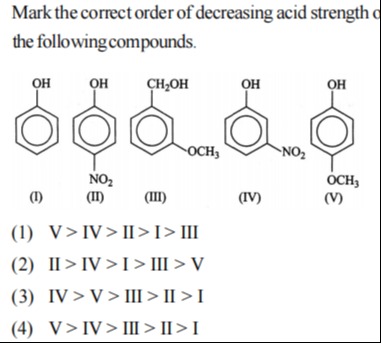
Mark the correct order of decreasing acid strength of the following compounds.
(I) (II) (III) (IV) (V)
A
V > IV > II > I > III
B
II > IV > I > III > V
C
IV > V > III > II > I
D
V > IV > III > II > I
Answer
II > IV > I > III > V
Explanation
Solution
– Nitro group (–NO₂) strongly withdraws electrons so nitrophenols [(II) and (IV)] are most acidic (with the p‐nitro isomer (II) being slightly stronger). – Phenol (I) is next. – In (V) the electron‑donating –OCH₃ group reduces acidity. – In (III) the –CH₂OH is aliphatic (pKₐ ~16) making it very weakly acidic.
