Question
Question: Which of the following changes exhibit that nitrogen undergoes oxidation?...
Which of the following changes exhibit that nitrogen undergoes oxidation?
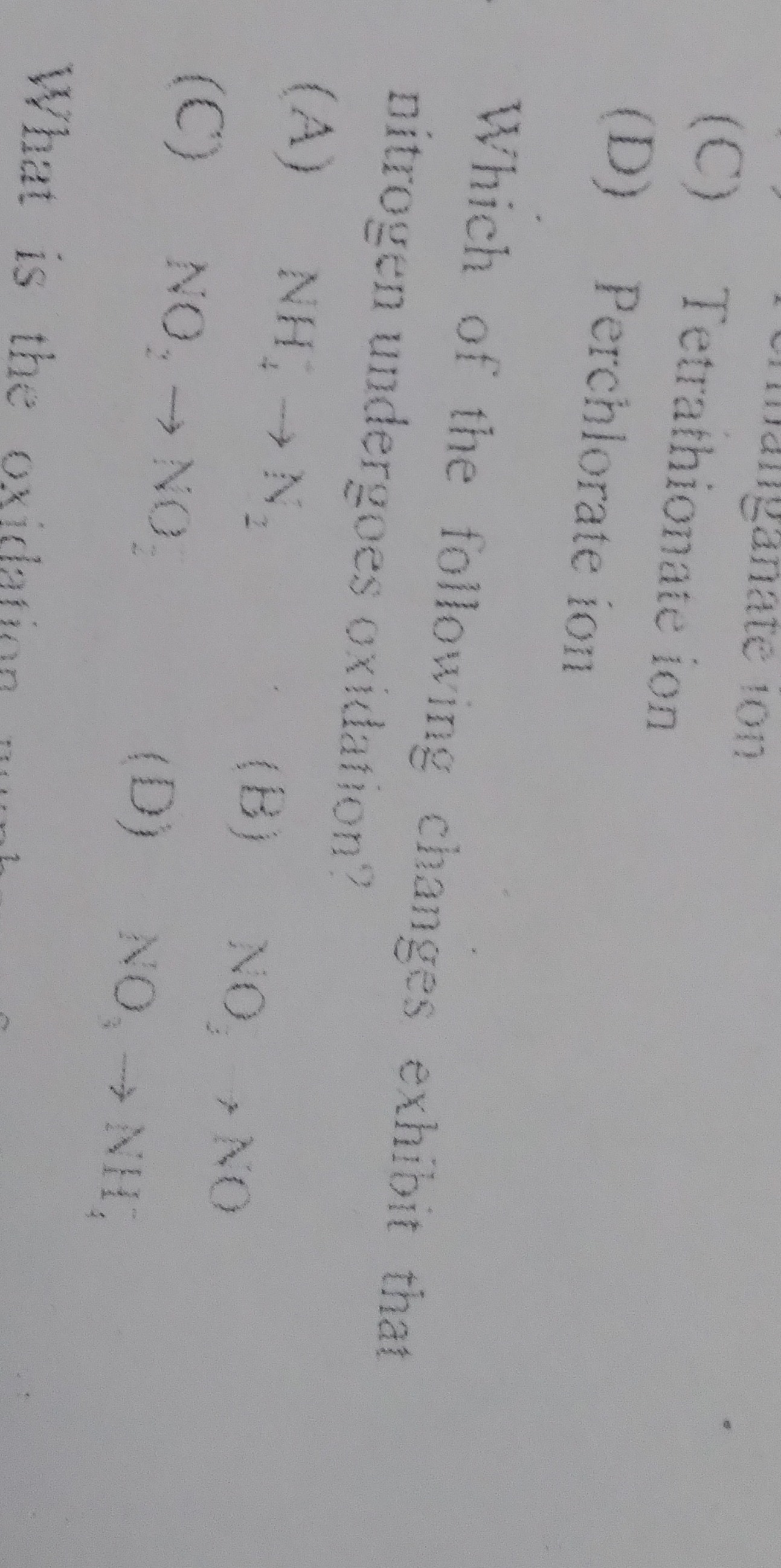
A
NH4→N2
B
NO2→NO
C
NO2→NO2
D
NO3→NH4
Answer
NH4→N2
Explanation
Solution
For oxidation, the oxidation state of nitrogen must increase.
- In (A): In NH4+, nitrogen has an oxidation state of −3; in N2, nitrogen has an oxidation state of 0.
- In (B): NO2 (N: +4) to NO (N: +2) is a reduction (decrease in oxidation state).
- (C) shows no change.
- In (D): NO3− (N: +5) to NH4+ (N: -3) is also a reduction.
Thus, only (A) exhibits oxidation of nitrogen.
Nitrogen in NH4+ goes from oxidation state −3 to 0 in N2, indicating oxidation.
