Question
Question: \( ( + ) - \) Mandelic acid has a specific rotation of \( + {158^o} \) . What would be the observed ...
(+)− Mandelic acid has a specific rotation of +158o . What would be the observed specific rotation of a mixture of 25%(−)− mandelic acid and 75%(+)− mandelic acid:
Solution
Mandelic acid is aromatic in nature and is alpha hydroxy acid. It has a molecular formula C6H5CH(OH)CO2H . It is a chiral molecule and its racemic mixture is known as para mandelic acid. Specific rotation is a property of a chemical compound which is chiral in nature. Now, we will find the observed specific rotation for the mandelic acid.
Complete answer:
So, now we know that Mandelic acid is aromatic in nature and is alpha hydroxy acid. It has a molecular formula C6H5CH(OH)CO2H . Its structure is:
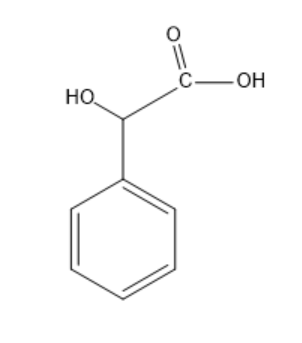
So we can clearly see that it is a chiral molecule and hence, shows specific rotation.
Specific rotation is defined as the change in the orientation of a monochromatic plane-polarized light as it passes through a sample of compound in the solution.
Formula used to find observed specific rotation is given as:
E.E=α(pure(+)enantiomer)[α]
Here, E.E refers to enantiomeric excess.
[α] is the observed specific rotation.
α is the specific rotation of pure (+)− Mandelic acid.
So, now we are given with:
Concentration of (−)− mandelic acid is 25% .
Concentration of (+)− Mandelic acid is 75% .
Enantiomeric excess of (+)− Mandelic acid is 75−25
=50%
Putting these values in the formula, we get:
50=158[α]×100%
[α]=+79o
Therefore, the observed specific rotation of the mixture is, [α]=+79o .
Note :
Chiral compounds are those compounds in which at least one carbon atom is chiral (have four different groups attached to it). Chiral compounds can rotate monochromatic plane-polarized light. Achiral molecules are those in which there is no chiral carbon present and this type of molecule is not able to rotate the plane-polarized light.
