Question
Question: Major product of the below reaction is: 
Solution
Hint: Here the conformer isomers are reacted with sodium ethoxide and there is a formation of two products which are cis and trans. Here, the reactant is interconverted by rotations by using a single bond. Here, sodium ethoxide acts as a strong base and it gives the product by following Zaitsev’s rule. And the trans product will be the major product that will govern the cis product due to less steric crowding.
Complete answer:
Here, N,N,N,2−tetramethyl−1−phenylpropan−1−aminium is reacted with sodium ethoxide in the presence of heat and there is a formation two products which cis and trans. And the trans product will be the major product and the product is, (E)-prop−1−ene−1,2−diyldibenzene. Here, the sodium ethoxide acts as a good nucleophile, and it will attack the substrate so that it is a stronger nucleophile and which is also a stronger base. Let’s see the chemical reaction,
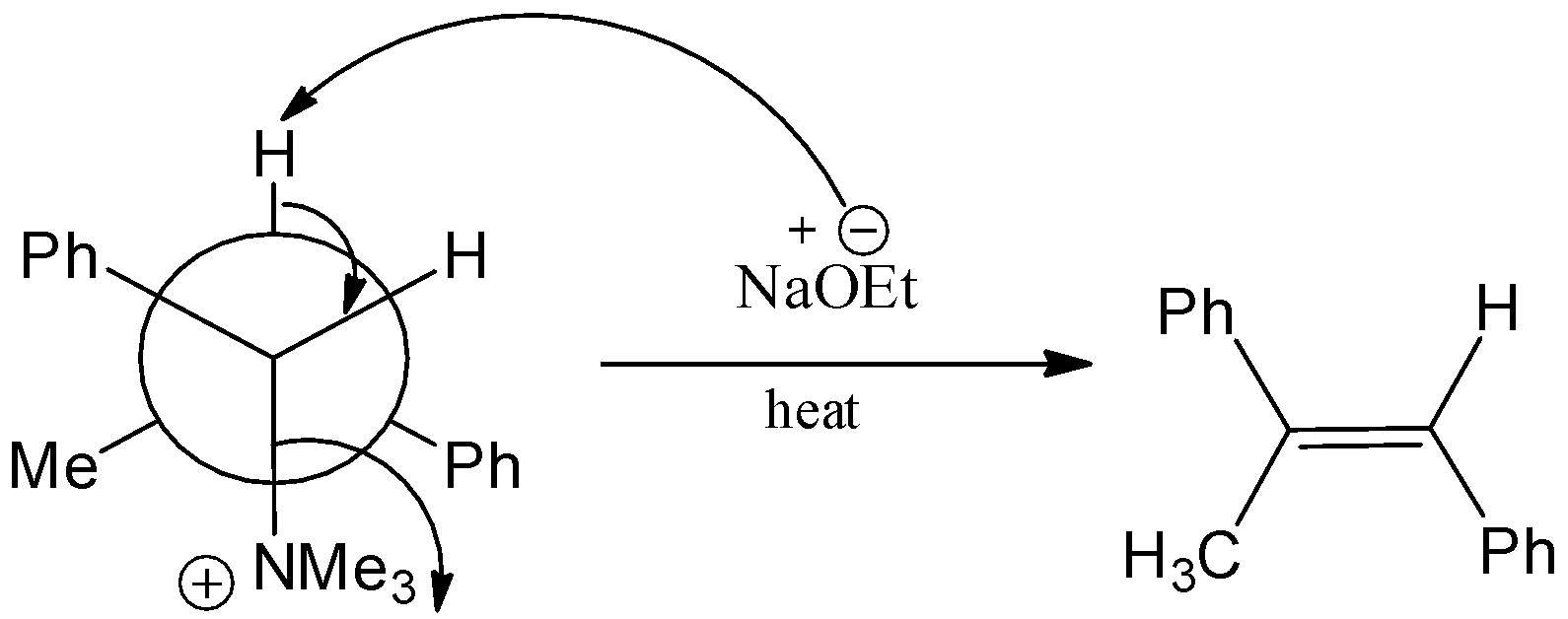
In sodium ethoxide, the ethoxide is the conjugate base of the corresponding alcohol. And the ethoxide acts as a string reducing agent. Here, the ethoxide group will first attack the hydrogen and protonation will take place. The trimethyl amine will be eliminated by the attack of ethoxide. Hence, there is a formation of a major product, which is (E)-prop−1−ene−1,2−diyldibenzene.
Note:
We must have to know that the conformer is defined as the isomer of the molecule, which has a difference from another isomer by rotation of a single bond that is present in the molecule. And these are formed by the confirmation process. And in this reaction, a trans product is formed as the major product due to the less steric crowding of trans products.
