Question
Question: Major product obtained during the following reaction sequence is:...
Major product obtained during the following reaction sequence is:
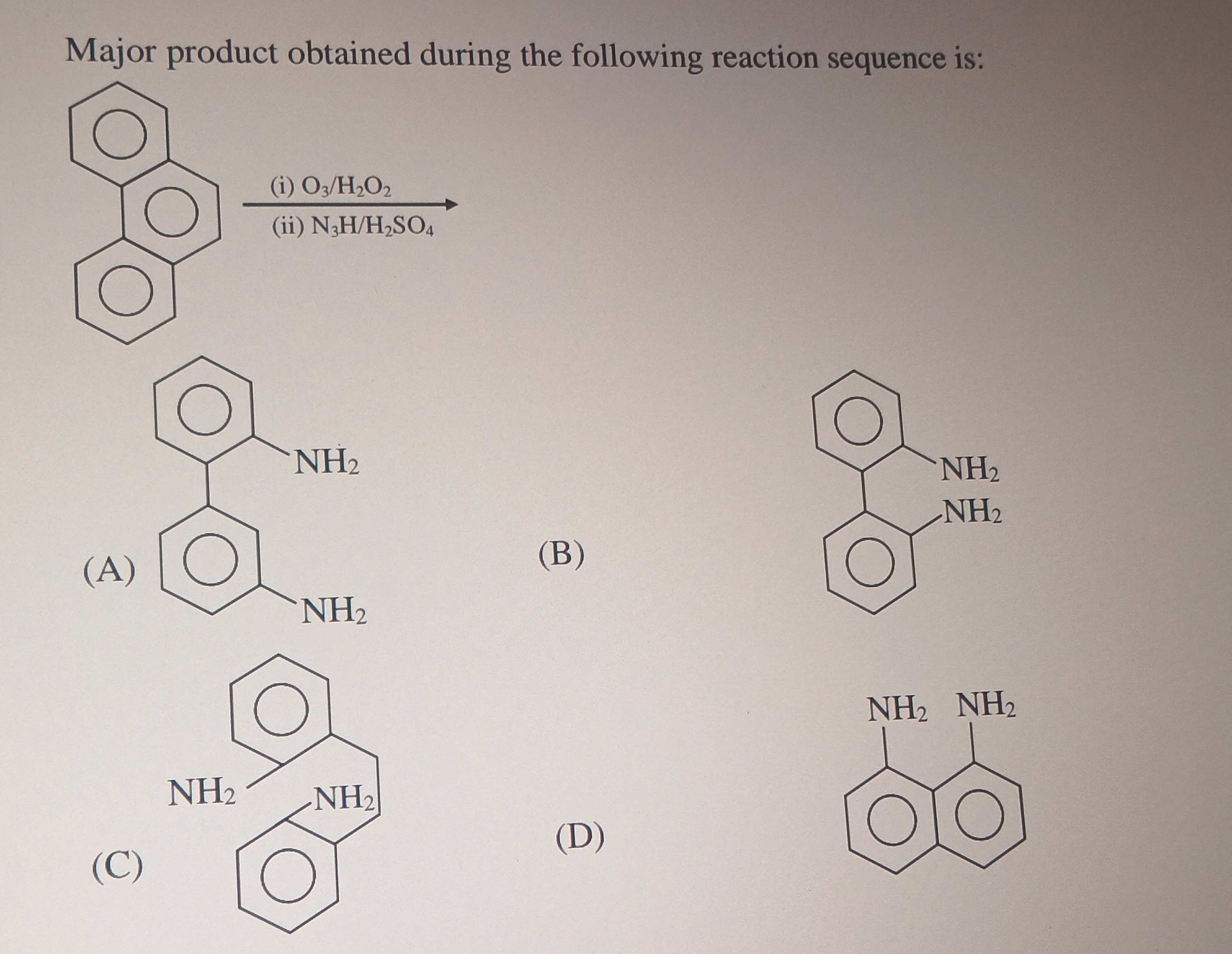
2,2'-diaminobiphenyl
2,3'-diaminobiphenyl
A saturated cyclic diamine
A derivative of naphthalene with two amino groups
2,2'-diaminobiphenyl
Solution
The reaction sequence begins with phenanthrene.
Step 1: Ozonolysis with oxidative workup (O3/H2O2) Phenanthrene undergoes ozonolysis at the 9,10 double bond. With oxidative workup (H2O2), this cleavage results in the formation of two carboxylic acid groups, yielding diphenic acid (2,2'-biphenyldicarboxylic acid).
The structure of diphenic acid is:
COOH
|
C1----C2
/ \ / \
C6 C C3
/ \ / \
C5------C4------C1'
\ / \ /
C---C---C---C
\ / \ /
COOH
Step 2: Reaction with hydrazoic acid and sulfuric acid (N3H/H2SO4) - Schmidt Reaction Diphenic acid, having two carboxylic acid groups, reacts with hydrazoic acid in the presence of sulfuric acid. This is the Schmidt reaction, where a carboxylic acid is converted into a primary amine with the loss of CO2.
Applying this to diphenic acid: Each -COOH group is converted to an -NH2 group.
The resulting product is 2,2'-diaminobiphenyl.
The structure of 2,2'-diaminobiphenyl is:
NH2
|
C1----C2
/ \ / \
C6 C C3
/ \ / \
C5------C4------C1'
\ / \ /
C---C---C---C
\ / \ /
NH2
This structure corresponds to option (A).
