Question
Question: Major product (A) is:  is:
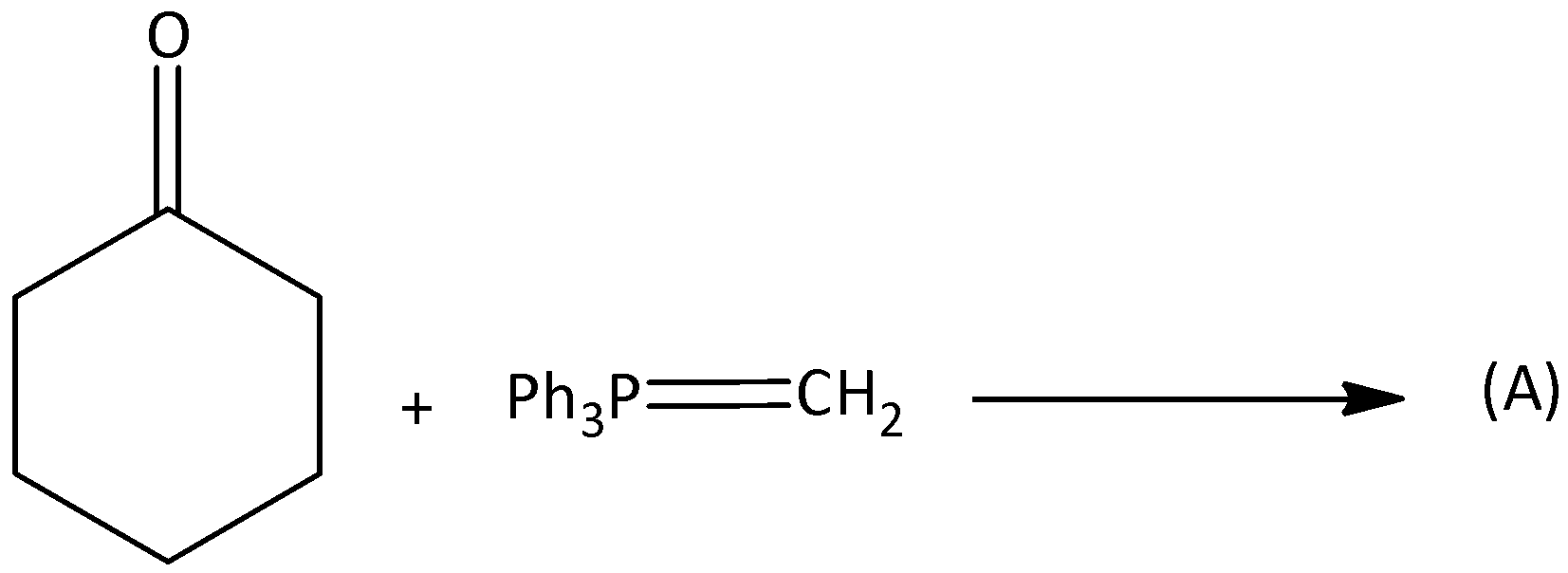
A.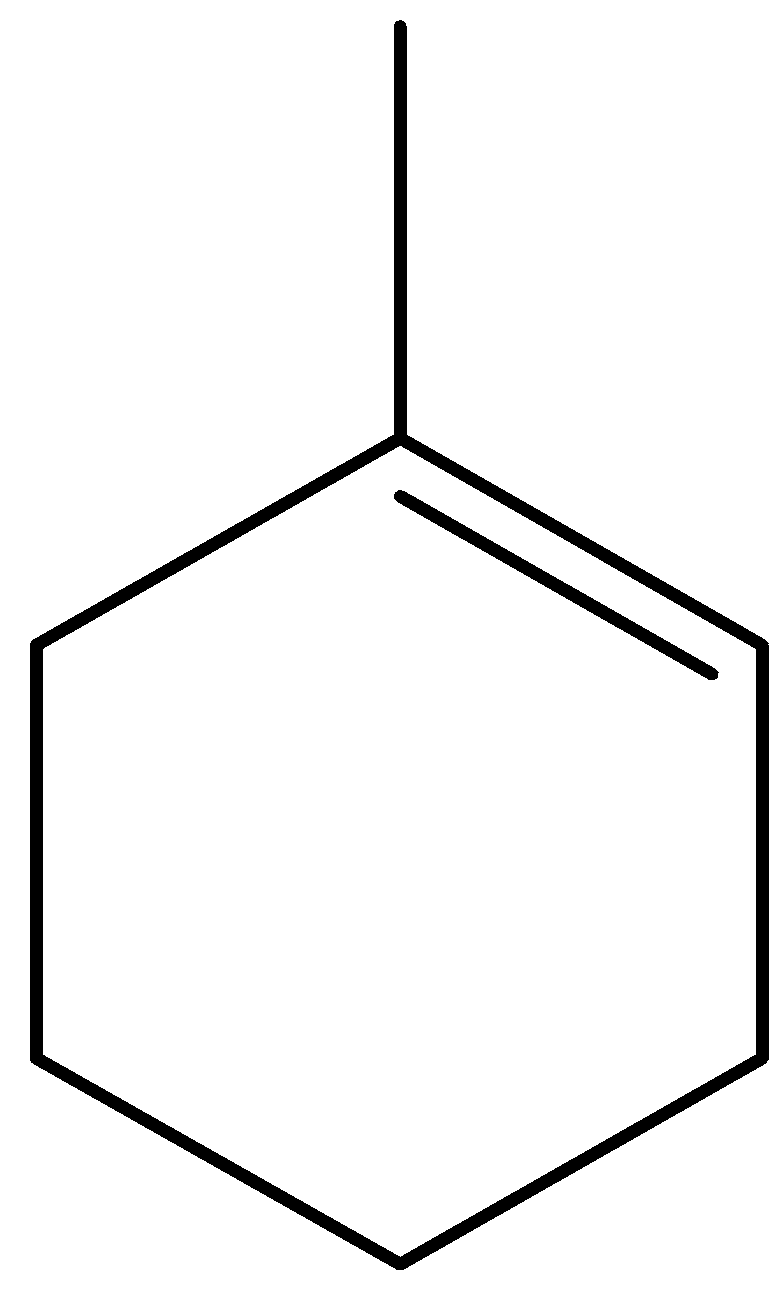
B.
C.
D.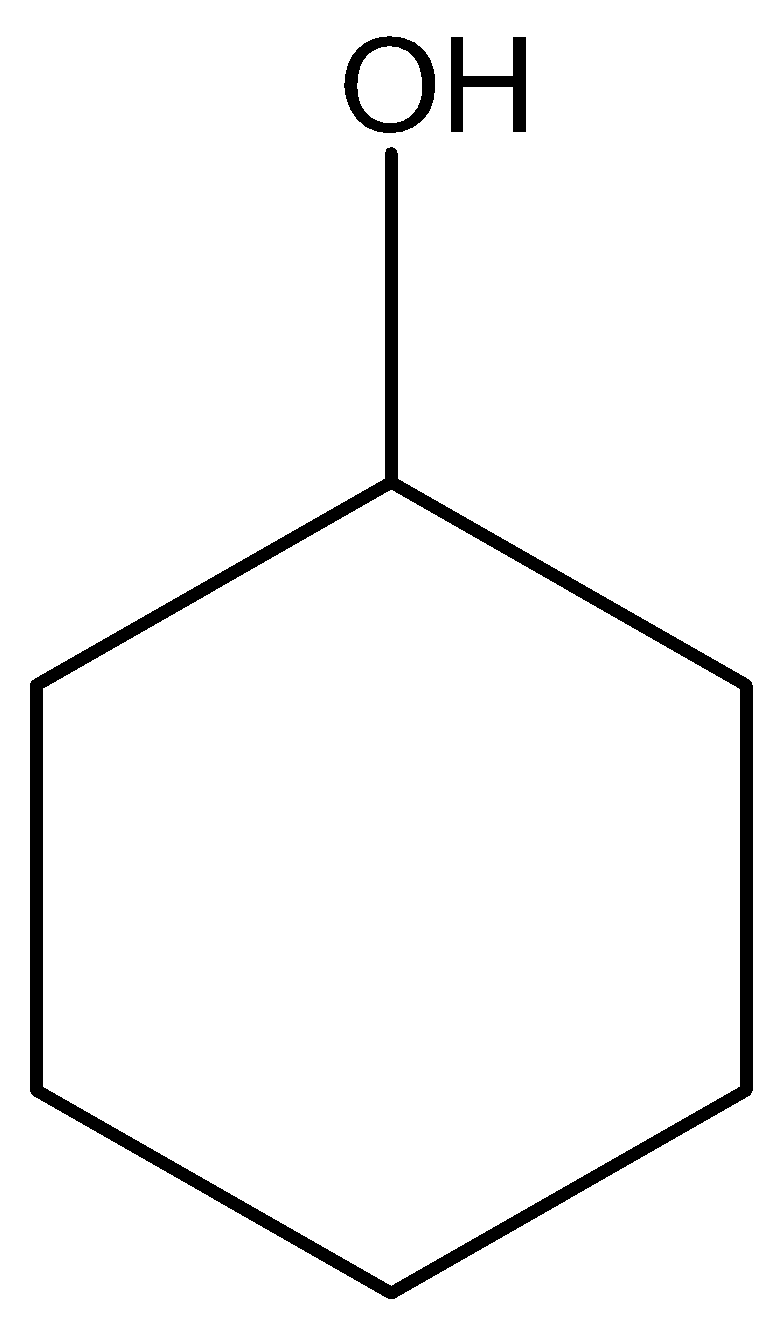
Solution
We can see that the one of the reactants is cyclohexanone, which is a ketone, and the other reactant is triphenyl phosphonium ylide. From the reactants, we can predict the reaction is the Wittig reaction. Triphenyl phosphonium ylide is known as the Wittig reaction. The product of the reaction should be alkene and triphenylphosphine oxide.
Complete answer: We have to remember that the Wittig reaction is otherwise known as Wittig Olefination. It is a coupling reaction, and is named after the scientist Georg Wittig.
We have to know that the Wittig reaction affords an important and useful method for the synthesis of alkenes by the treatment of aldehydes or ketones with alkylidene triphen phosphorane or simply known as phosphorane.
We can use the Wittig reaction for the production of alkenes.
Now coming back to question, cyclohexanone reacts with methylene phenyl phosphorane to form alkene and triphenylphosphine oxide. Methylene Phenyl Phosphorane is the chief member of the phosphorus ylides. Methylene Phenyl Phosphorane is otherwise called as Wittig reagents. This reagent is a highly basic species, and is highly polar.
When cyclohexanone reacts with methylene phenyl phosphorane, the products formed are 1-methylcyclohex-1-ene and methylenecyclohexane. We can write this reaction as,
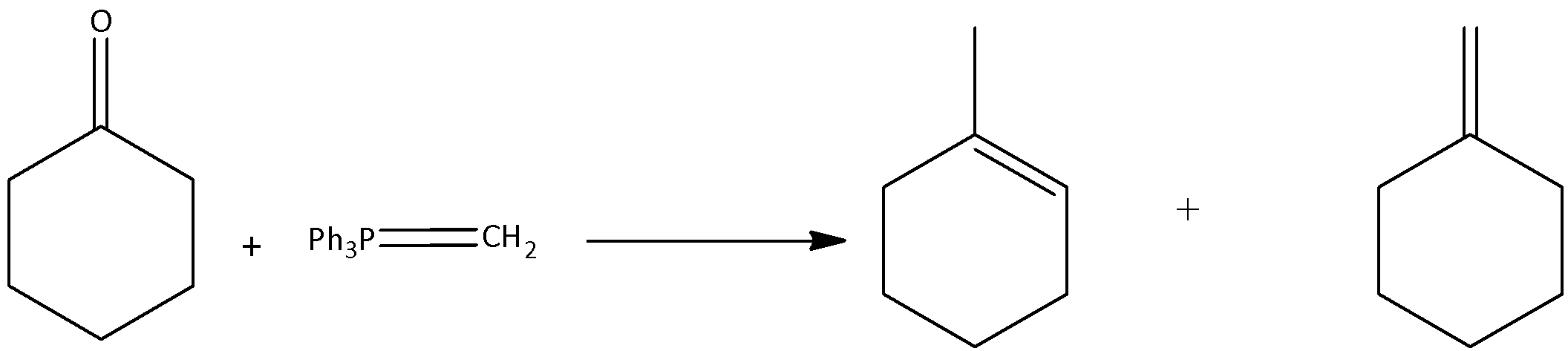
Triphenylphosphine oxide is formed as the minor product.
The correct options are (A) and (C).
Some of the advantages of Wittig reaction are,
-Through Wittig reaction alkenes are prepared from aldehydes and ketones.
-By the Wittig reagent acceptance of carbonyl compounds with many kinds of functional groups like OH group, OR group, etc.
-Once the nature of ylides is known the geometry of the double bond can easily be predicted.
Note: One should not confuse between Wittig rearrangement and Wittig reaction. Wittig reaction could be used for monosubstituted, disubstituted, and trisubstituted alkenes. The product of Wittig reaction is a pure alkene with known structure. The typical solvents that are used in Wittig reagent are tetrahydrofuran (or) diethyl ether.
