Question
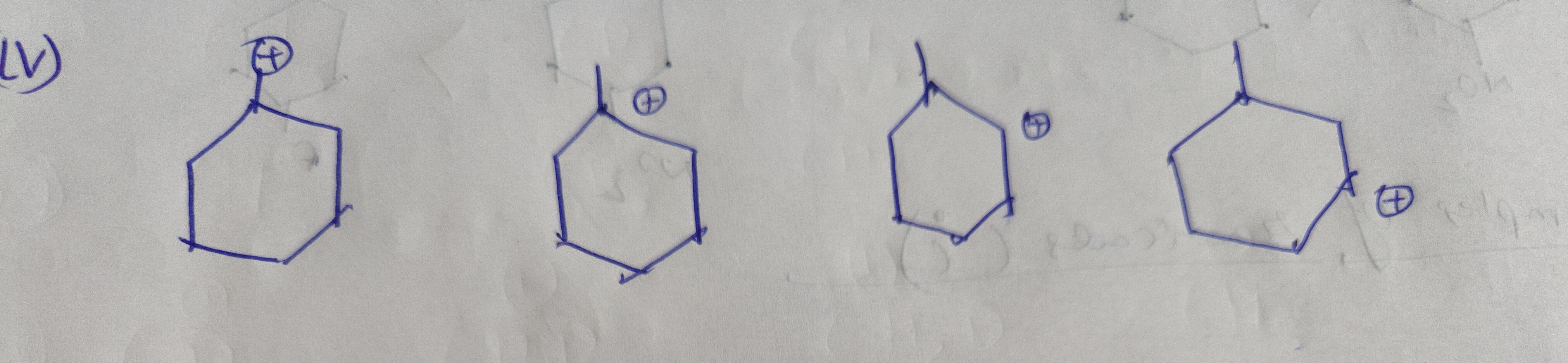
Question: The provided image displays four cyclohexane carbocations. The question is not explicitly stated, bu...
The provided image displays four cyclohexane carbocations. The question is not explicitly stated, but it is highly probable that it concerns the relative stability of these carbocations.
Analysis of Carbocations:
All four structures represent secondary carbocations, as the positive charge is located on a carbon atom bonded to two other carbon atoms. The stability of carbocations is influenced by several factors:
- Degree of Substitution: Tertiary carbocations are more stable than secondary, which are more stable than primary.
- Resonance: Carbocations that can be resonance-stabilized (e.g., allylic, benzylic) are significantly more stable.
- Substituent Effects: Electron-donating groups (EDGs) stabilize carbocations through inductive effect and hyperconjugation. Electron-withdrawing groups (EWGs) destabilize carbocations.
Let's examine each structure:
-
Structure 1, 2, and 3: These are unsubstituted secondary carbocations within a cyclohexane ring. Their stability is expected to be similar, as there are no significant electronic differences or resonance stabilization. The different orientations of the rings in the drawings likely represent different conformations (e.g., chair), but for a simple secondary carbocation, electronic factors are usually dominant.
-
Structure 4: This is also a secondary carbocation, but it has a substituent labeled 'A' on the adjacent carbon atom. The effect of 'A' on the stability of the carbocation depends on its electronic nature.
- If 'A' is an electron-donating group (e.g., an alkyl group like methyl, ethyl; or groups like -OH, -NH₂, -OR), it will stabilize the carbocation through inductive effect and hyperconjugation, making structure 4 more stable than the unsubstituted secondary carbocations (1, 2, and 3).
- If 'A' is an electron-withdrawing group (e.g., a halogen, -NO₂, -CN), it will destabilize the carbocation, making structure 4 less stable than the unsubstituted secondary carbocations.
Assumed Question and Solution:
Assuming the question asks to rank the carbocations in order of decreasing stability, and assuming 'A' represents a common electron-donating group like a methyl group (CH₃), the stability order would be:
Structure 4 (secondary carbocation with adjacent EDG) > Structures 1, 2, 3 (unsubstituted secondary carbocations).
The relative stability of structures 1, 2, and 3 is considered to be very similar.
Therefore, the order of decreasing stability is: 4 > 1 ≈ 2 ≈ 3.
If the question were "Which of the following carbocations is the most stable?", the answer would be structure 4 (assuming 'A' is an EDG).
If the question were "Which of the following carbocations are equally stable?", and assuming 1, 2, and 3 are considered equally stable, then the answer would be 1, 2, and 3.
Given the lack of explicit question and options, we will provide the most likely interpretation related to ranking stability.
Structure 4 is the most stable.
Structures 1, 2, and 3 are equally stable and more stable than structure 4.
Structure 4 is the least stable.
Structures 1, 2, and 3 are equally stable and less stable than structure 4.
Structure 4 is the most stable.
Solution
Carbocation stability is enhanced by electron-donating groups and resonance. All structures are secondary carbocations. Structure 4 has an adjacent substituent 'A'. Assuming 'A' is an electron-donating group, it stabilizes the carbocation, making structure 4 the most stable. Structures 1, 2, and 3 are unsubstituted secondary carbocations of similar stability.
