Question
Question: Lower alcohols are highly soluble in water due to A) Van der Waals attraction B) Dipole dipole...
Lower alcohols are highly soluble in water due to
A) Van der Waals attraction
B) Dipole dipole interaction
C) Steric hindrance
D) H-bonding
Solution
The solubility of alcohol in water is due to the formation of hydrogen bond. More easily the hydrogen bond is formed more will be the solubility.
Complete answer:
The lower alcohols are soluble in water but as the number of alkyl groups increases or as the molecular mass increases the solubility decreases. We know that the solubility of alcohols in water is due to the formation of hydrogen bonds with water. The extent of solubility totally depends upon the ability of alcohols to form hydrogen bonding with the water molecules.
As the molecular mass of the hydrocarbon part increases ,the non-polar part increases among the alcohols which offers resistance towards the formation of hydrogen bonding with water. Therefore the solubility goes down as the number of carbon chains increases.MSo we can say that lower alcohols are soluble in water due to the formation of hydrogen bonding with the water molecule.
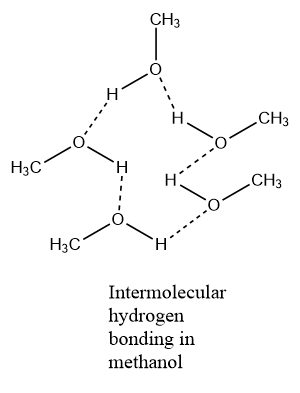
Hence the correct answer is option (D).
Note: It should be noted that among isomeric alcohols the solubility is increased due to the formation of branching. This is due to the fact that as branching increases the surface area of the non polar part that is the hydrocarbon part decreases and hence it offers less resistance towards hydrogen bonding, therefore solubility increases.
