Question
Question: Low spin complex of d5-cation in an octahedral field will have the following energy: (A) \[-2{{\D...
Low spin complex of d5-cation in an octahedral field will have the following energy:
(A) −2Δ0+P
(B) −2Δ0+2P
(C) 5−2Δ0+2P
(D) −2Δ0+3P
Solution
The formula to give the Crystal Field Splitting Energy (CFSE) of a cation in an octahedral field is
No. of electrons in t2g×(−0.4)Δ0+no. of electrons in eg×(0.6)Δ0+electron pairs×pairing energy(P)
Try and apply this theory to the given scenario.
Complete step by step solution:
Let us first try and understand the Crystal Field Theory which governs the phenomenon required for us to give a correct answer in the given question.
The Crystal Field Theory (CFT) is a model for the bonding interaction between transition metals and ligands. It describes the effect of the attraction between the positive charge of the metal cation and negative charge on the non-bonding electrons of the ligand. When the ligands approach the central metal ion, the degeneracy of electronic orbital states, usually d or f orbitals, are broken due to the static electric field produced by a surrounding charge distribution.
The electrons in the d orbitals of the central metal ion and those in the ligand repel each other due to repulsion between like charges. Therefore, the d electrons closer to the ligands will have higher energy than those further away, which results in the d orbitals splitting in energy.
The d orbitals, as we know, can be divided into two smaller sets. The dx2−y2 and dz2 orbitals point directly along the axes. On the other hand, the lobes of the dxy, dyz and dzx orbitals lie between any two axes which are very evident from their symbols. When a ligand approaches these degenerate orbitals, this orientation of atomic orbitals splits them into two sets. The eg set comprises of dx2−y2 and dz2 orbitals while the t2g set includes dxy, dyz and dzx orbitals. In the case of octahedral complexes, the crystal field splitting results into the egset to be higher in energy while t2g set is lower in energy. The total crystal field splitting energy is represented as Δo where “o” in the subscript stands for octahedral complexes. The t2g and eg sets are not evenly split from the base energy value, which is also known as the barycentre, of the atomic orbitals. The values and the splitting are clear in the diagram below:
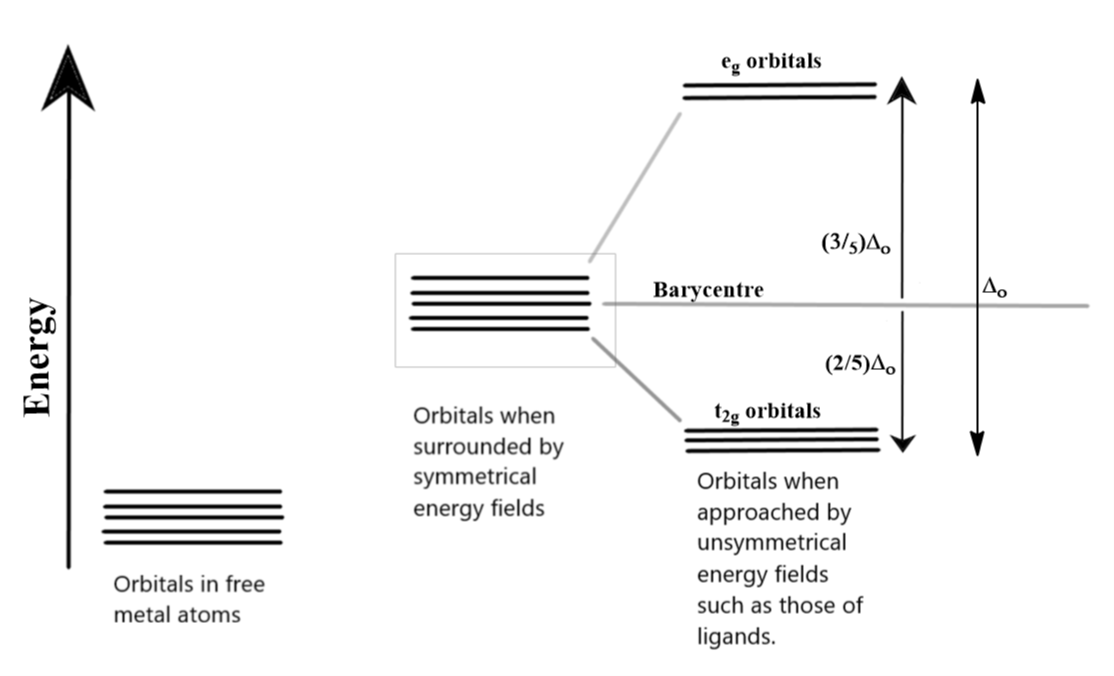
A low spin complex is formed when the next electron entering these split orbitals in the configuration of d4 and onwards does not jump into the higher eg orbitals but pairs up with an existing electron in the lowert2g orbitals. This happens only when the crystal field splitting energy (Δo) is higher than the pairing energy “P”. So the electronic configuration becomes t2g4eg0. This goes on until the configuration becomes t2g6eg0, after which the electrons start jumping up into higher energy d-orbitals because of the lack of space in the lower energy orbitals.
The configuration given here is d5, so a low-spin complex would look like: t2g5eg0. Therefore, by the given formula, the Crystal Field Splitting Energy (CFSE) here is given by:
