Question
Question: Light from a discharge tube containing hydrogen atoms falls on a piece of sodium due to the transiti...
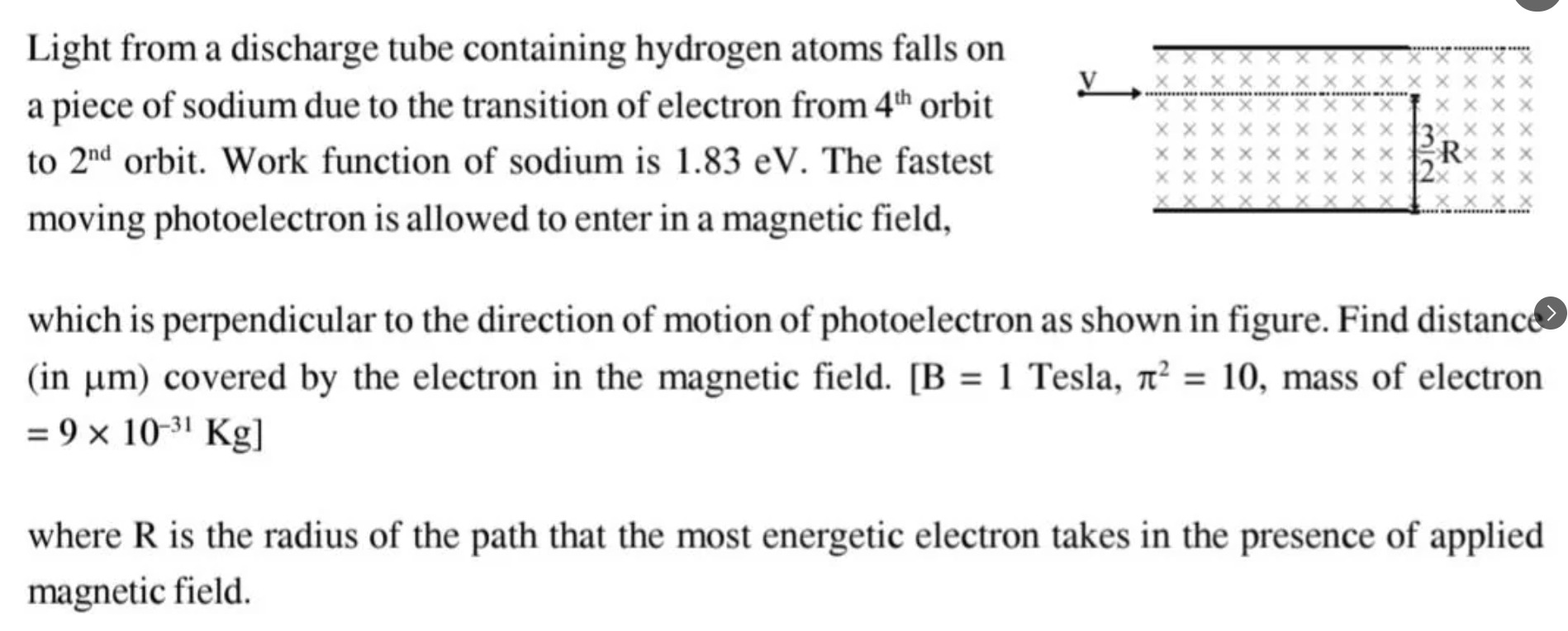
Light from a discharge tube containing hydrogen atoms falls on a piece of sodium due to the transition of electron from 4th orbit to 2nd orbit. Work function of sodium is 1.83 eV. The fastest moving photoelectron is allowed to enter in a magnetic field, which is perpendicular to the direction of motion of photoelectron as shown in figure. Find distance (in µm) covered by the electron in the magnetic field. [B = 1 Tesla, π² = 10, mass of electron = 9 × 10-31 Kg]
6
Solution
The energy of the photon emitted from the hydrogen atom due to the transition of the electron from the 4th orbit to the 2nd orbit is given by: Ephoton=13.6(nf21−ni21) eV Here, ni=4 and nf=2. Ephoton=13.6(221−421)=13.6(41−161)=13.6(164−1)=13.6×163=1640.8=2.55 eV.
The work function of sodium is ϕ=1.83 eV. The maximum kinetic energy of the photoelectron is given by Einstein's photoelectric equation: KEmax=Ephoton−ϕ=2.55 eV−1.83 eV=0.72 eV.
Convert the kinetic energy to Joules: KEmax=0.72 eV×1.6×10−19 J/eV=1.152×10−19 J.
The kinetic energy is also given by KEmax=21mv2. 21mv2=1.152×10−19 v2=m2×1.152×10−19=9×10−312.304×10−19=0.256×1012. v=0.256×1012=0.256×106=1000256×106=100016×106=101016×106=101.6×106. Given π2=10, so 10=π. v=π1.6×106 m/s.
The photoelectron enters a magnetic field perpendicular to its velocity. The electron follows a circular path with radius R given by: R=eBmv Given m=9×10−31 Kg, e=1.6×10−19 C, B=1 T, and v=π1.6×106 m/s. R=(1.6×10−19)×1(9×10−31)×(π1.6×106)=1.6×10−19×π9×10−31×1.6×106=π×10−199×10−25=π9×10−6 m. R=π9 µm.
The angle subtended by the arc at the center is θ=π−3π=32π.
The distance covered by the electron in the magnetic field is the arc length, which is s=Rθ. s=R×32π. We have R=π9 µm. s=π9×32π=39×2=3×2=6 µm.
