Question
Question: Lewis structure of \[{\rm{Be}}{{\rm{F}}_{\rm{2}}}\] 
(A) True
(B) False
Solution
As we know that, Lewis acids are electron deficient compounds which do not have complete octet. Beryllium is the element of alkaline earth metal and fluorine is the element of the halogen family.
Complete solution
Lewis represented the atom as positively charged kernel and the outer shell. The outermost shell is occupied by the eight electrons. Thus, these electrons in the outermost shell take part in chemical combinations in Lewis structure. Lewis structures are represented by Lewis symbols or electron dot symbols. In this structure of BeF2, valence electrons are represented by electron dot symbols.
As we know that, the beryllium atom is the member of the second group in the periodic table and it has two electrons in its outermost shell. Therefore, these electrons only participate in bond formation with fluorine. So, one electron of each fluorine forms bonds with two electrons of beryllium.
This is shown below
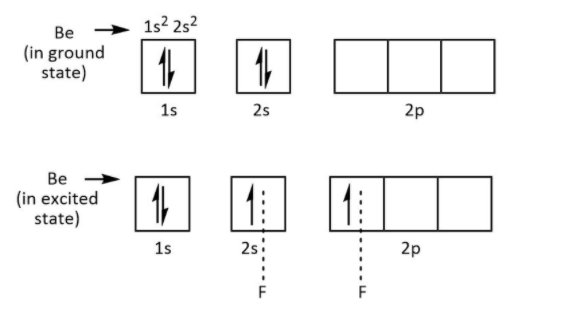
So only four electrons are available in the structure of BeF2, it needs four electrons to complete its octet. That’s why BeF2 also known as Lewis acid.
The actual structure is represented as-
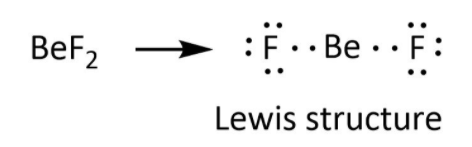
**Therefore, the correct option for the given statement is option (B) that is False.
Note: **
The above structure is the exception of Lewis octet rule, in which octet is incomplete but there are several molecules or compounds which have more than eight electrons in the outermost shell of central atom such as SF6 has twelve electrons around the central atom, PF5 has ten and IF7 has fourteen electrons. These molecules are stable.
