Question
Question: Let us assume that one mole of an ideal diatomic gas undergoes a transition from A to B along a path...
Let us assume that one mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as represented in the diagram. What will be the variation in internal energy of the gas at the time of transition?
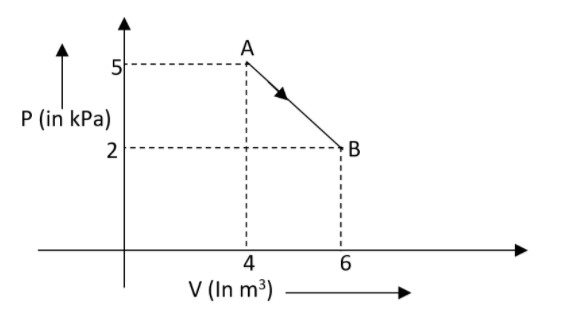
A.20JB.−12kJC.20kJD.−20kJ
Solution
The change in internal energy can be found by taking the product of the number of moles of the gas, the specific heat capacity at constant volume and the change in temperature. Find the temperature for each condition using the ideal gas equation. Substitute this in the change in internal energy equation. This will help us to solve this question.
Complete step by step answer:
The change in internal energy can be found by taking the product of the number of moles of the gas, the specific heat capacity at constant volume and the change in temperature.
That is we can write that,
ΔU=nCVΔT
Where n be the number of moles, CV be the specific heat capacity at constant volume and ΔT be the change in temperature.
For an ideal diatomic gas, the specific heat capacity at constant volume will be,
CV=23R
From the ideal gas equation we can write that,
T=RPV
Where P be the pressure, V be the volume and R be universal gas constant.
This can be applied in both the conditions such as,
