Question
Question: Let the ground state electronic energy of hydrogen atom be $E_H$ eV. The first excited state energy ...
Let the ground state electronic energy of hydrogen atom be EH eV. The first excited state energy of deuterium atom (in eV) is
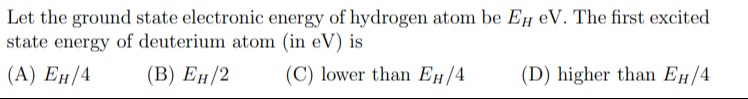
EH/4
EH/2
lower than EH/4
higher than EH/4
lower than EH/4
Solution
The energy of an electron in a hydrogen-like atom is En∝−n2μ, where μ is the reduced mass. The reduced mass μ=me+MmeM increases with the nuclear mass M. Since the deuteron mass (Md) is greater than the proton mass (Mp), the reduced mass for deuterium (μD) is greater than that for hydrogen (μH).
The ground state energy of hydrogen is EH=−meμHRy∞.
The first excited state energy of deuterium is ED,n=2=−4meμDRy∞.
Comparing ED,n=2 with EH/4=−4meμHRy∞.
Since μD>μH, it follows that 4meμD>4meμH. Therefore, −4meμDRy∞<−4meμHRy∞, which means ED,n=2 is lower than EH/4.
