Question
Question: \(\left( {{\text{N}}{{\text{H}}_{\text{3}}}} \right)\) has much higher boiling point than \(\left( {...
(NH3) has much higher boiling point than (PH3) because:
A. (NH3)has much higher molecular mass.
B.(NH3) forms hydrogen bonds.
C.(NH3) contains ionic bond while (PH3) contains covalent bonds.
D. (NH3)undergoes umbrella inversion
Solution
We know that hydrogen bond is a type of bond that is present in molecules and this bond is also written as H – bond. To understand a hydrogen bond we consider a molecule that has an H atom say HA. In the molecule HA, A is a strongly electronegative element and H atom linked with A by a normal covalent bond. Electron pairs that are present in the molecule will be shared between H and A atom. Thus H will be partially positive and A will be partially negative. HA molecule thus behaves as a dipole that is represented by H δ + −Aδ −. Now another molecule say HB also forms dipole Hδ + −Bδ − that is brought near Hδ + −Aδ −. These two dipoles are linked by a type of bond which is also called hydrogen bond. Hydrogen bond between two molecule is shown as follows:
Hδ + −Aδ −........Hδ + −Bδ −
Complete step by step answer:
As we know that ammonia (NH3) molecule is smaller than phosphine (PH3) molecule. The dispersion force of ammonia molecules is smaller than the phosphine molecule.
NH3 molecule has a strong hydrogen bond between N and H atoms. This leads to intermolecular force and thus has a greater attraction between NH3 . Due to this reason NH3 has much higher boiling point than PH3. Hydrogen bond between NH3 molecule is shown is as follows:
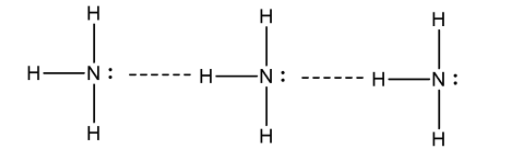
Hence, the option B is the correct answer
Note:
Hydrogen bonds are classified as intramolecular H bonding and intermolecular hydrogen bonding. In intramolecular hydrogen bonding H occurs between a single molecule and in inter molecule hydrogen bonding H occurs between a more similar or different molecule.
