Question
Question: \( {\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]^{3 + }} \) is paramagnetic while \( {\left[ {Ni...
[Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2− is diamagnetic. Explain why?
Solution
Hint : We solve this using valence bond theory. Before solving this we need to know the type of ligand that is weak field ligand or strong field ligand. If it is a weak field ligand then it cannot pair up the electron and if it is a strong field ligand then it can pair up the electron. In the central metal atom if we have an unpaired electron then it is paramagnetic. In the central metal atom if we have paired electrons then it is diamagnetic.
Complete step by step solution:
We have,
[Cr(NH3)6]3+
We need the oxidation state of Chromium Cr .
We know that NH3 is a neutral ligand so that the oxidation state is zero.
Cr+6(0)=+3
Cr=+3
That is the oxidation state is 3.
We know that the atomic number of Chromium is 24.
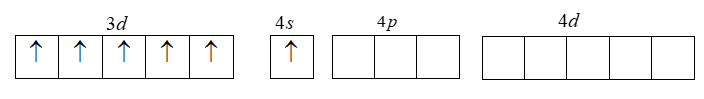
The electronic configuration of Cr is given by
[Ar]3d54s14p04d0 .
(these cannot be done in latex form)
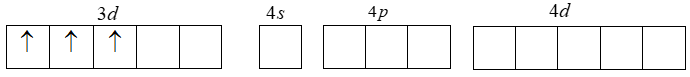
The electronic configuration of Cr+3 is given by
[Ar]3d34s04p04d0 . That is loss of one ‘4s’ electron and two ‘3d’ electrons. Thus the resulting Cr+3 ion has outer electronic configuration of 3d3 .
Now we can see the hybridization
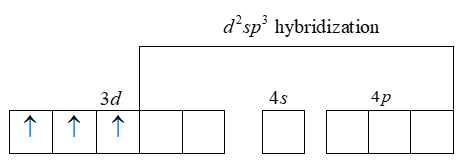
Thus we have two vacant 3d-orbital, one 4s-orbital and three 4p-orbitals hybridise to give six equivalent d2sp3 hybrid orbitals. Six pairs of electrons, one from each NH3 molecule occupy the six vacant hybrid orbitals which are produced.
Now let’s see electronic configuration of [Cr(NH3)6]3+ complex.
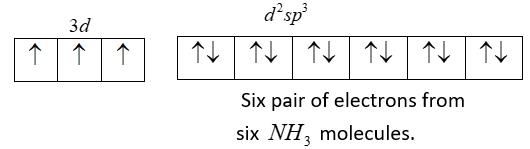
We can see that we have unpaired electrons so it is paramagnetic.
Now [Ni(CN)4]2−
We need the oxidation state of Nickel Ni .
We know that CN is a strong ligand so that the oxidation state is −1 .
Ni+4(−1)=−2
Ni=+2
That is the oxidation state is 2.
We know that the atomic number of Chromium is 27.
The electronic configuration of Ni is given by
[Ar]3d84s24p04d0 .
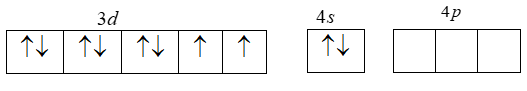
The electronic configuration of Ni+2 is given by
[Ar]3d84s04p0 . That is the loss of two ‘4s’ electrons. Thus the resulting Ni+2 ion has outer electronic configuration of 3d3 .
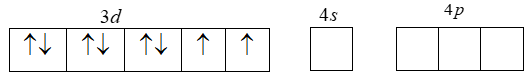
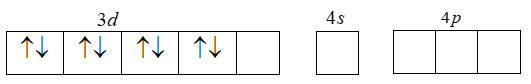
Also that CN is a strong ligand and pairing of electrons takes place. That is the two unpaired 3d-electrons are forced to pair up.
Since we have four CN ligands which donate 4 pairs of electrons resulting in dsp2 hybrid orbitals and from each cyanide ion occupy the four vacant hybrid orbitals.
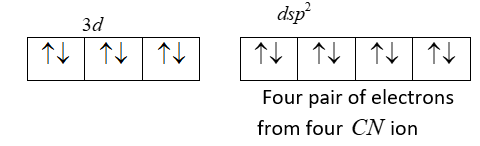
We can see that there is no unpaired electron. So it is diamagnetic.
Note:
[Cr(NH3)6]3+ is d2sp3 hybridization hence the geometry of the complex ion is octahedral. [Ni(CN)4]2− is dsp2 hybridization hence the geometry of complex ion is square planar. Similarly if we have sp hybridization then the geometry is linear. If we have sp2 hybridization then the geometry is triangular planar. If we have sp3 hybridization then the geometry is tetrahedral.
