Question
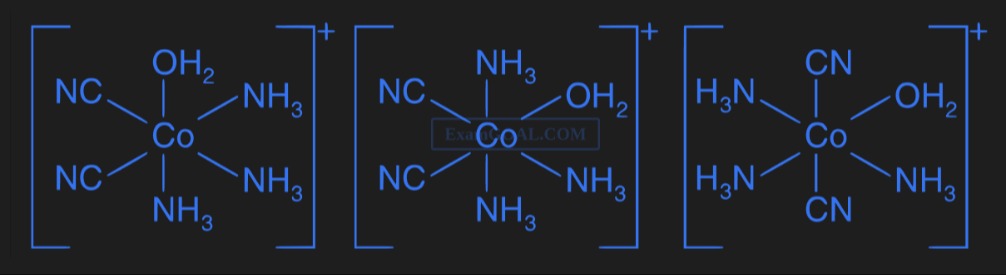
Question: $\left[ \begin{array}{c} \text{NC} \hspace{10mm} \text{OH}_2 \\ \text{NC} - \text{Co} - \text{NH}_3...
NCOH2NC−Co−NH3NH3+
NH3OH2NC−Co−NH3NCNH3+
H3NCNH3N−Co−OH2H3NCN+
Answer
Geometric isomers
Explanation
Solution
The three given complexes have the same chemical formula, [Co(CN)2(NH3)3(H2O)]+ but different spatial arrangements of ligands around the central cobalt ion. This difference in arrangement, while maintaining the same connectivity, defines them as geometric isomers.
