Question
Question: \(\lbrack FeF_{6}\rbrack^{3 -}\)is paramagnetic due to presence of unpaired electrons in the complex...
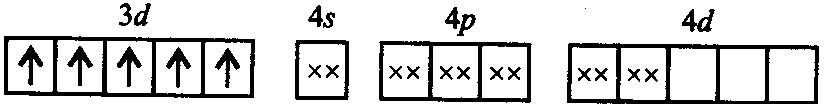
[FeF6]3−is paramagnetic due to presence of unpaired electrons in the complex. The five electrons remains unpaired because
A
Fluorine is the most electronegative element
B
F−is a weak field ligand hence does not cause pairing of electrons
C
F−is a strong field ligand hence does not cause pairing of electrons
D
Pairing does not take place in iron complexes.
Answer
F−is a weak field ligand hence does not cause pairing of electrons
Explanation
Solution
F−is weak field ligand hence it does no cause pairing of electrons.
[FeF6]3−: