Question
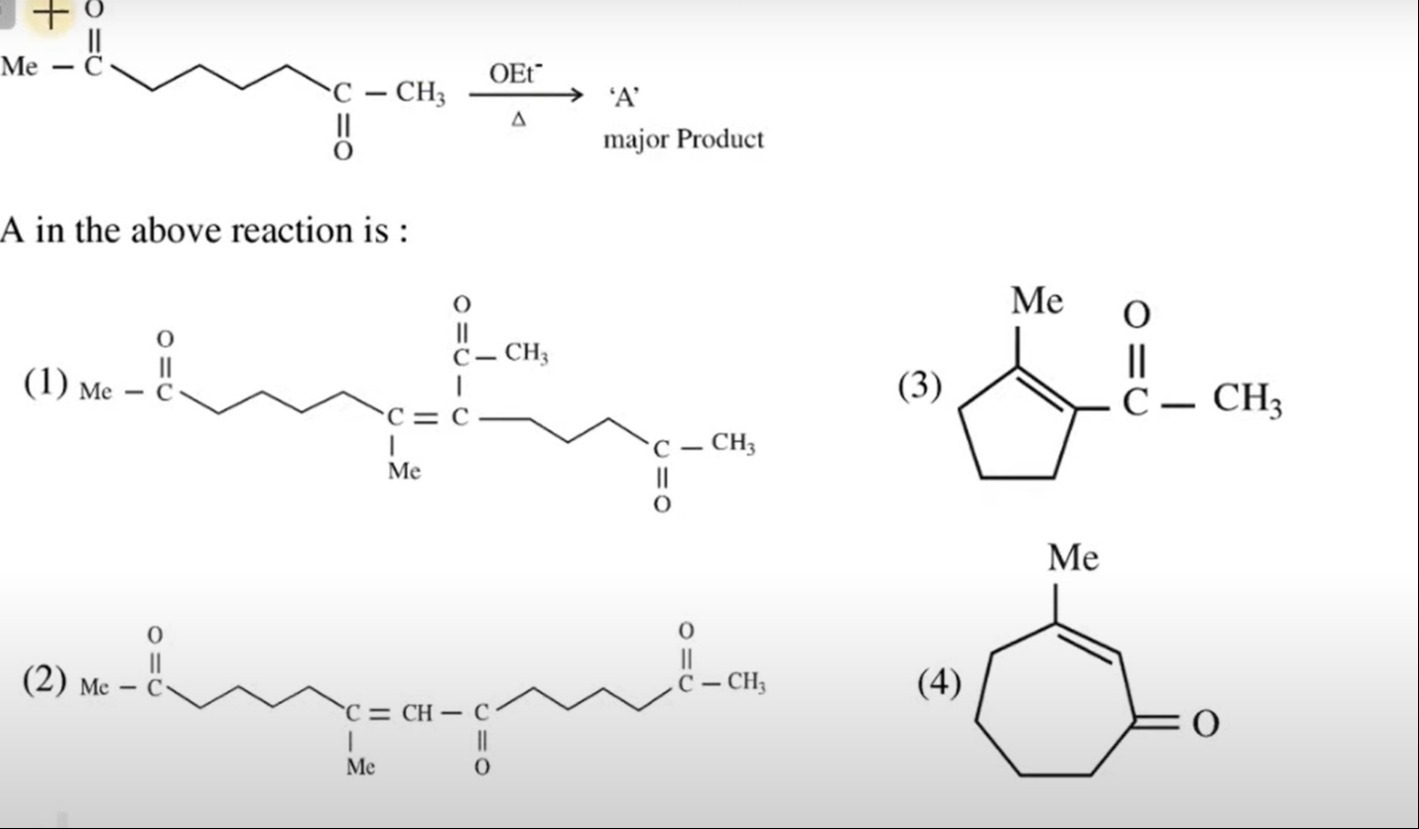
Question: Me-C=O C=O -CH3 $\xrightarrow[\Delta]{\text{OEt}^-}$ 'A' major Product A in the above reaction is...
Me-C=O C=O -CH3
OEt−Δ 'A' major Product
A in the above reaction is :
\begin{array}{c} \text{CH}_3-\text{C}=\text{O} \\ \text{CH}_2-\text{CH}_2 \\ \text{CH}_2-\text{CH}_2 \\ \text{CH}-\text{CH}_3 \end{array}
\begin{array}{c} \text{CH}_3-\text{C}=\text{O} \\ \text{CH}_2-\text{CH}_2 \\ \text{CH}_2-\text{CH}_2 \\ \text{C}-\text{CH}_3 \end{array}
\begin{array}{c} \text{CH}_3-\text{C}=\text{O} \\ \text{CH}_2-\text{CH}_2 \\ \text{C}=\text{C}-\text{CH}_3 \\ \text{C}-\text{C}(=\text{O})\text{CH}_3 \end{array}
\begin{array}{c} \text{CH}_3-\text{C}=\text{O} \\ \text{CH}_2-\text{CH}_2 \\ \text{CH}_2-\text{CH}_2 \\ \text{CH}_2-\text{C}=\text{CH}_3 \end{array}
\begin{array}{c} \text{CH}_3-\text{C}=\text{O} \\ \text{CH}_2-\text{CH}_2 \\ \text{C}=\text{C}-\text{CH}_3 \\ \text{C}-\text{C}(=\text{O})\text{CH}_3 \end{array}
Solution
The reaction is an intramolecular aldol condensation of a 1,7-diketone. The reactant is 2,7-octanedione: CH3−CO−(CH2)4−CO−CH3.
The base (OEt−) and heat (Δ) promote the formation of an enolate, which then attacks an intramolecular carbonyl group. There are two possible modes of enolate formation:
-
Formation of a 5-membered ring: Deprotonation of a methylene group (α-carbon adjacent to a carbonyl) leads to an enolate that attacks the other carbonyl group, forming a 5-membered ring. This pathway is generally favored due to the higher stability of 5-membered rings.
- Enolate formation at C3 (or C6) of CH3−CO−CH2−CH2−CH2−CH2−CO−CH3.
- The enolate attacks the carbonyl at C7.
- This forms a 5-membered ring (C3−C4−C5−C6−C7).
- The intermediate is a cyclic β-hydroxy ketone.
- Dehydration of this intermediate leads to an α,β-unsaturated ketone. The most stable product is formed when the double bond is conjugated with the ketone. This results in the formation of 2-acetyl-3-methylcyclopent-2-en-1-one, which corresponds to option (3).
-
Formation of a 7-membered ring: Deprotonation of a methyl group (α-carbon) leads to an enolate that attacks the other carbonyl group, forming a 7-membered ring.
- Enolate formation at C1 (or C8) of CH3−CO−CH2−CH2−CH2−CH2−CO−CH3.
- The enolate attacks the carbonyl at C7.
- This forms a 7-membered ring (C1−C2−C3−C4−C5−C6−C7).
- Dehydration leads to a conjugated system.
Since 5-membered ring formation is generally favored over 7-membered ring formation in intramolecular aldol reactions, the major product is the one arising from the 5-membered ring cyclization.
The structure of option (3) is: A 5-membered ring with a ketone group (C=O) and a methyl group (CH3) attached to one carbon of the ring. Adjacent to the ketone is a double bond within the ring. One carbon of the double bond has a methyl group, and the other carbon of the double bond has an acetyl group (C(=O)CH3). This structure is consistent with the product formed via the 5-membered ring pathway.
