Question
Question: ```latex \begin{array}{c} \text{CHO}\\ \text{ |}\\ \text{(CHOH)}_4 \xrightarrow{\text{Reagent(R)}} ...
\begin{array}{c} \text{CHO}\\ \text{ |}\\ \text{(CHOH)}_4 \xrightarrow{\text{Reagent(R)}} \text{Product (P)}\\ \text{ |}\\ \text{CH}_2\text{OH} \end{array}
Choose the correct statement regarding 'R' and 'P'.
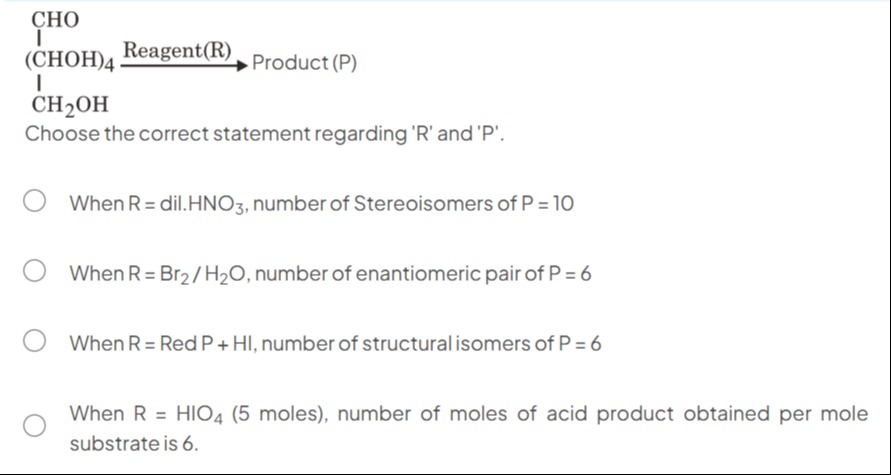
When R = dil.HNO3, number of Stereoisomers of P = 10
When R = Br2/H2O, number of enantiomeric pair of P = 6
When R = Red P + HI, number of structural isomers of P = 6
When R = HIO4 (5 moles), number of moles of acid product obtained per mole substrate is 6.
Options 1 and 4 are correct.
Solution
Solution:
We start with an aldo‐hexose (CHO–(CHOH)₄–CH₂OH). In dilute HNO₃ the sugar is oxidized at both ends to give the corresponding hexaric (mucic) acid. A standard result is that aldohexaric acids exist as 10 stereoisomers.
On the other hand, oxidation by periodic acid (HIO₄, used in 5‐mole quantity per mole sugar) cleaves all the vicinal diols so that, on further oxidation, every carbon is converted into an acid group – a net yield of 6 moles of carboxylic acid per mole of sugar.
The other two statements (regarding Br₂/H₂O and Red P + HI reactions) do not give the numbers indicated.
Explanation (minimal):
- R = dil. HNO₃: The sugar is oxidized at both ends giving an aldaric (mucic) acid. It is known that such compounds have 10 stereoisomers.
- R = HIO₄ (5 moles): Complete oxidative cleavage converts each carbon of the sugar into an acid group so that one mole sugar yields 6 moles of acid.
- The other reagents lead to different transformations than those stated.
