Question
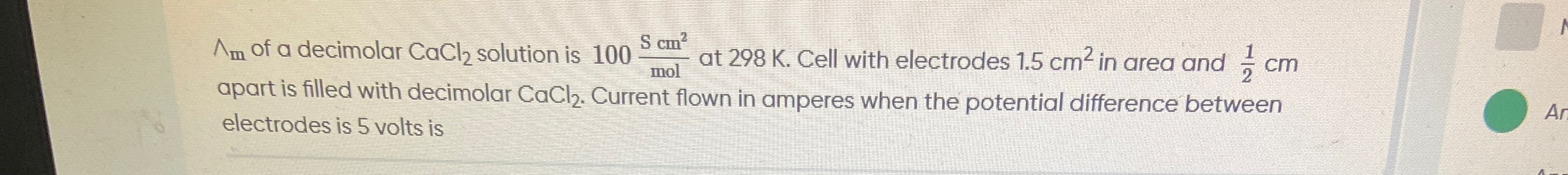
Question: $\Lambda_m$ of a decimolar $CaCl_2$ solution is $100 \frac{S cm^2}{mol}$ at 298 K. Cell with electro...
Λm of a decimolar CaCl2 solution is 100molScm2 at 298 K. Cell with electrodes 1.5 cm2 in area and 21 cm apart is filled with decimolar CaCl2. Current flown in amperes when the potential difference between electrodes is 5 volts is
0.15
Solution
To calculate the current flowing through the electrochemical cell, we follow these steps:
-
Calculate the Cell Constant (G∗): The cell constant is defined as the ratio of the distance between the electrodes (l) to the area of the electrodes (A). Given: l=0.5 cm, A=1.5 cm2 G∗=Al=1.5 cm20.5 cm=31 cm−1
-
Calculate the Specific Conductivity (κ): The molar conductivity (Λm) is related to the specific conductivity (κ) and the concentration (C) by the formula: Λm=Cκ×1000 Where Λm is in S cm2 mol−1, κ is in S cm−1, and C is in mol L−1. Given: Λm=100 S cm2 mol−1, C=0.1 M=0.1 mol L−1 Rearranging the formula to find κ: κ=1000Λm×C κ=1000100 S cm2 mol−1×0.1 mol L−1 κ=100010 S cm−1 κ=0.01 S cm−1
-
Calculate the Conductance (G): Conductance (G) is related to specific conductivity (κ) and cell constant (G∗) by the formula: G=G∗κ G=1/3 cm−10.01 S cm−1 G=0.01×3 S G=0.03 S
-
Calculate the Current (I): According to Ohm's Law, the current (I) is given by the potential difference (V) divided by the resistance (R). Since conductance (G) is the reciprocal of resistance (R=1/G), we can write: I=RV=V×G Given: V=5 volts I=5 V×0.03 S I=0.15 A
The current flown in amperes is 0.15 A.
