Question
Question: Kjeldahl’s method cannot be used for: This question has multiple correct options A. \(PhN{{O}_{2...
Kjeldahl’s method cannot be used for:
This question has multiple correct options
A. PhNO2
B. PhNHCOPh
C. Azobenzene
D. Pyridine
Solution
The answer is based on the fact that Kjeldahl’s method is used for the determination of nitrogen in the organic and inorganic substances and find out which compounds cannot be converted to ammonium sulfate and those compounds will be the correct options.
Complete step by step answer:
In the lower classes, we have carried out several experiments to determine the elements present in the compound and are familiar with these concepts.
Let us discuss in detail Kjeldahl’s method which is also used for the determination of one of the elements by considering each option given.
- Kjeldahl’s method in analytical chemistry is the method used for the quantitative determination of nitrogen contained in organic substances and also nitrogen present in the inorganic compounds like ammonia and ammonium.
- This method basically involved heating of the sample to around 360-410 degrees with the concentrated H2SO4 which then decomposes by the oxidation to liberate the reduced nitrogen in organic substance as ammonium sulfate.
- This method is not used for the nitro groups, azo groups, or for the nitrogen present in the ring.
- In option A) Nitrobenzene(PhNO2), when treated with sulphuric acid, it does not give out ammonium sulfate and this method is not suitable for the nitrogen which is present in the ring. Thus, this option is correct.
- In option B) PhNHCOPh that is benzanilide, the nitrogen here is not present in the ring and thus this forms ammonium sulfate where this method can be used to determine the nitrogen. The structure is,
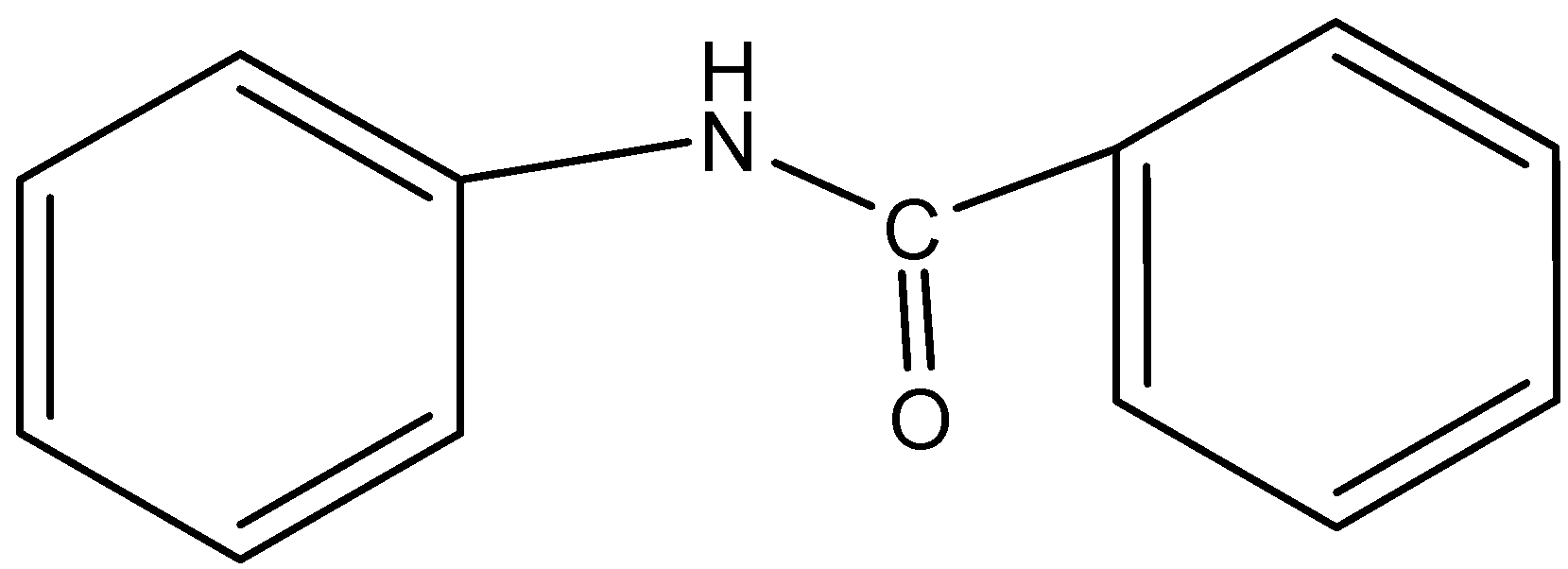
Therefore, this option is ruled out.
- In option C) Azobenzene which has the nitrogen as azo form does give a test for Kjeldahl’s method this option is correct. The structure of azobenzene is,
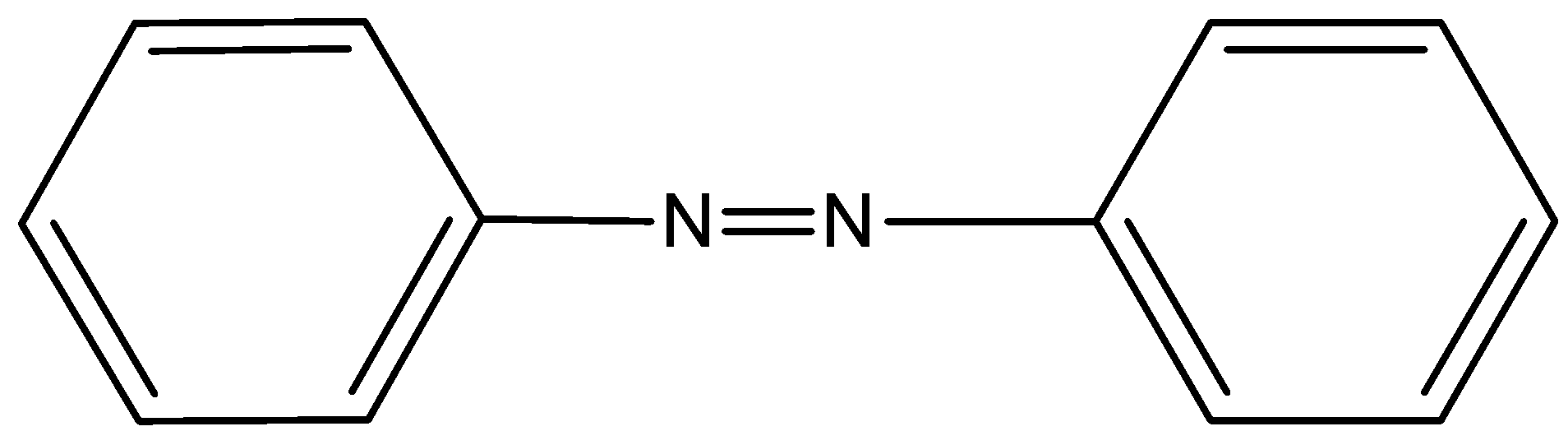
- In option D) Pyridine which is having the nitrogen present in the ring cannot be used for the determination of nitrogen as it cannot form ammonium sulfate. The structure of pyridine is
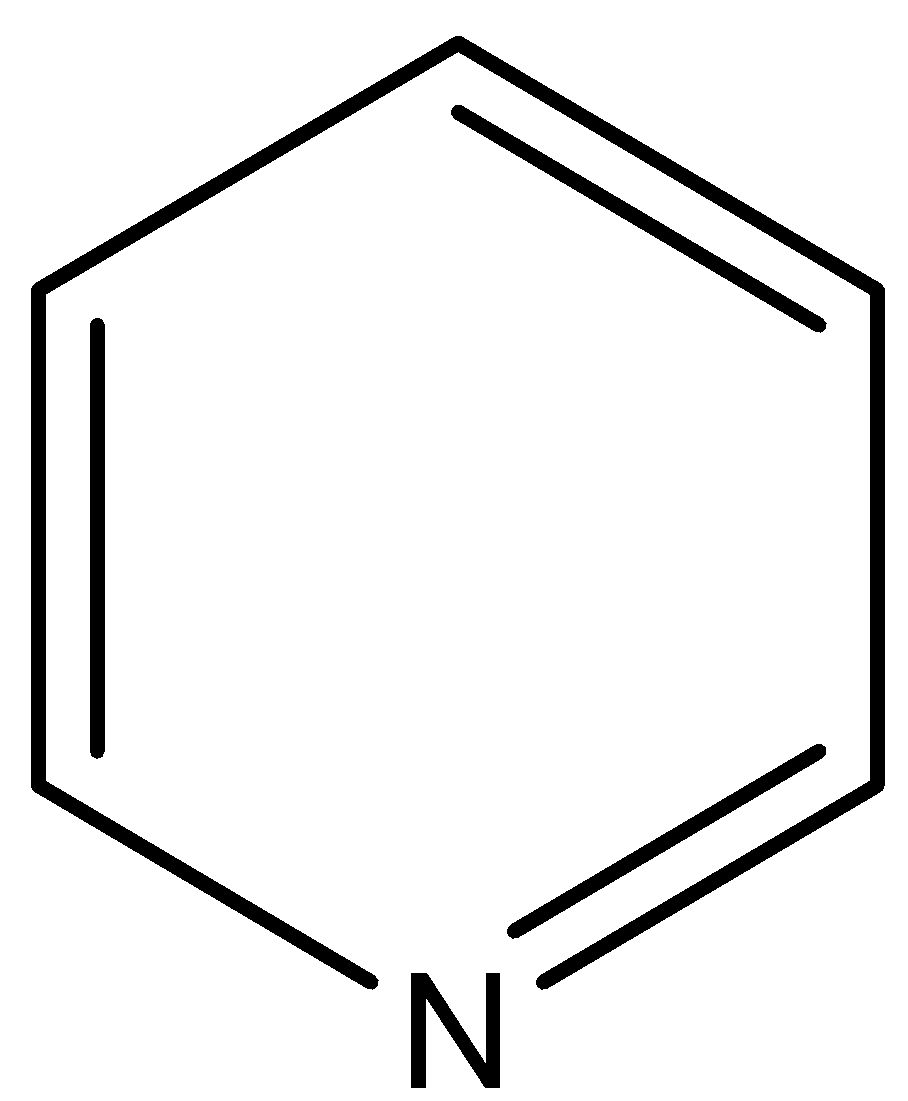
Note: Note that Kjeldahl’s method in the determination of nitrogen in protein does not give the actual content of nitrogen present in protein and hence another method called as Dumas method is used for the determination of nitrogen.
