Question
Question: Kinetic theory of matter states about: A) Three states of matter B) Difference between three sta...
Kinetic theory of matter states about:
A) Three states of matter
B) Difference between three states of matter
C) Fourth state of matter
D) All of the above
Solution
Kinetic theory of matter considers the composition of matters and states about the properties of matter such as pressure, volume, temperature. It also tells us that the particles are present in a large number in the matter.
Complete answer:
 Kinetic theory of gases states that the cause of pressure is the collision between the molecules of matter and with the container. Pressure is not caused by the molecules pushing each other away. Three main components of kinetic theory:
Kinetic theory of gases states that the cause of pressure is the collision between the molecules of matter and with the container. Pressure is not caused by the molecules pushing each other away. Three main components of kinetic theory:
1. There is no loss of energy when the molecules collide.
2. The molecules of gas take a very small space in comparison with the container
3. The motion of molecules is linear and constant.
As we have seen in the above explanation that the kinetic theory explains that the whole of the matter is made up of moving molecules. So, now we will understand the explanation for three states separately.
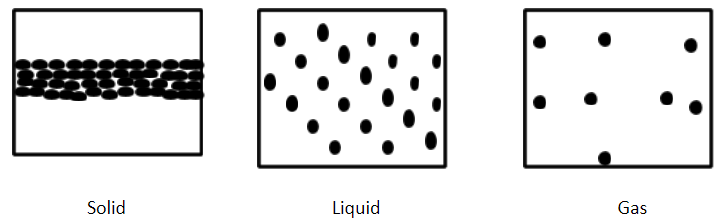
In solids, the particles are bound to each other very tightly. So the particles in solids cannot move from their location. The particles can vibrate only on their location.
In liquids, the particles have enough free space to move but the particles attract each other.
In gases, the particles are far apart from each other and have enough space to move freely.
Result: Above explanation shows that the kinetic theory of matter states about the difference between three states of matter.
So, option (B) is correct.
Note: Kinetic theory of matter describes the microscopic properties of atoms and it gives the description of macroscopic properties like volume, pressure and temperature. Kinetic theory of matter tells us about three states of matter and it also tells us how one state changes into another. The temperature of a matter gives the average kinetic energy of the particles of that matter.
