Question
Question: Keto-enol tautomerism is observed in: A.) \({C_6}{H_5} - CHO\) B.) \({C_6}{H_5} - CO - C{H_3}\) ...
Keto-enol tautomerism is observed in:
A.) C6H5−CHO
B.) C6H5−CO−CH3
C.) C6H5−CO−C6H5
D.) C6H5−CO−C(CH3)2C6H5
Solution
This question can be solved by the concept that Keto-enol tautomerism is observed in that molecules in which α−hydrogen is present that is the hydrogen attached to first carbon which is attached to the functional group.
Complete step by step answer:
The tautomerism is the structural isomerism of chemical compounds that are interconvertible. They differ from each other only in the position of a hydrogen atom and a double bond. Keto-enol tautomerism is one of its type. In Keto-enol tautomerism, there is chemical equilibrium between a keto group (an aldehyde or a ketone) and an enol group (an alcohol). The keto group can be an aldehyde functional group or a ketone functional group and enol group is the alcohol functional group. The keto- enol tautomerism can be represented by the example of acetone as given below:
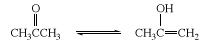
The Keto-enol tautomerism occurs due to the acidity of alpha hydrogens. Therefore, we can say that the Keto-enol tautomerism is shown by the compounds that are having α−hydrogens attached to them. The alpha hydrogen is the hydrogen which is attached to the alpha carbon and alpha carbon is that carbon which is the first carbon which is attached next to the carbon of the functional group.
In the given option only option B.) has alpha hydrogen in it that is in C6H5−CO−CH3 there are three alpha hydrogen.
Hence, option B.) is the correct answer.
Note:
Always remember that this question can also be directly solved by the concept that if there is a methylene group (−CH2−) is attached to the carbonyl group (⟩C=O) then it shows Keto-enol tautomerism.
