Question
Question: Keto-enol tautomerism is not observed in: /*This question has multiple correct options*/ a.) Phe...
Keto-enol tautomerism is not observed in:
/This question has multiple correct options/
a.) Phenol
b.) Glycerol
c.) HCN
d.) Benzophenone
Solution
Hint: Keto-enol tautomerism is defined as the chemical equilibrium of the ketone or aldehyde with an alcoholic group. For a tautomerism transformation to take place, the compound must have an α- hydrogen atom.
Complete step-by-step answer:
The aldehyde form and the alcoholic form are known as tautomers of each other. Tautomers are basically the isomers formed by the compounds which can be differentiated only with the position of protons and electrons. The carbon body of tautomers is strongly bounded and cannot be separated easily as they have fixed skeleton structure positions. For example, NO2(Nitrogen dioxide).
In a normal reaction of tautomerism, enol consists of carbon atoms adjacent to (OH) group and a carbon-carbon bond with OH group at equilibrium state. For example, it can be seen between vinyl alcohols(CH2CHOH) and acetaldehydes (CH3CHO) when a chemical reaction occurs between them at equilibrium state.
The molecular structures for Phenol and glycerol are given below –
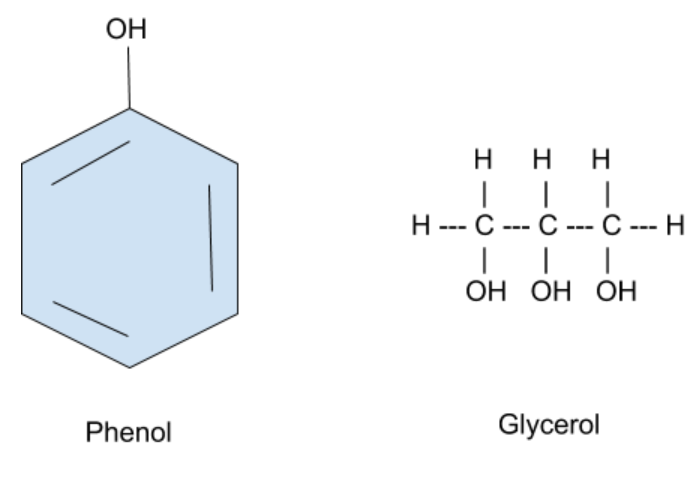
As we can see, both these compounds have an α- hydrogen atom and therefore, Keto-enol tautomerism can be observed in both of them.
The molecular structures for HCN and Benzophenone are given below –
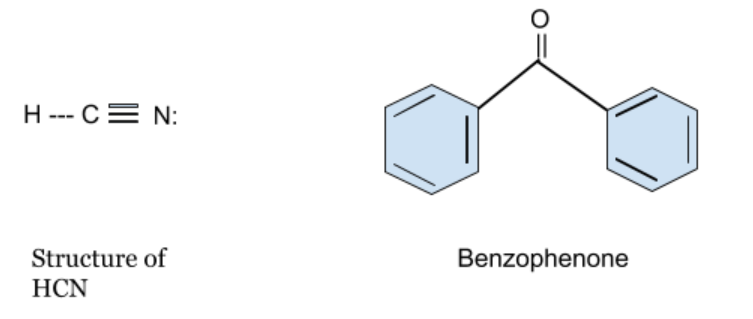
In this question, the hydrogen cyanide and the Benzophenone both don't have any α- hydrogen atom. That’s why Keto-enol tautomerism cannot be observed in both
Hence, the correct options are (C) and (D).
NOTE- Keto-enol tautomerism is the chemical equilibrium between the keto aka aldehyde form and the enol aka alcohol form. The tautomers must have anα- hydrogen atom in order to maintain a state of equilibrium. The compounds which do not have this atom cannot show keto-enol tautomerism. This is an important point to keep in mind while solving questions of keto-enol tautomerism.
