Question
Question: \({K_2}C{r_2}{O_7}\) when reacts with cold conc. \({H_2}S{O_4}\) gives red crystal of A \(Cr{O_4}...
K2Cr2O7 when reacts with cold conc. H2SO4 gives red crystal of
A CrO42−
B CrO3
C Cr2(SO4)3
D Cr2O3
Solution
Potassium dichromate that is K2Cr2O7is a good oxidising agent and also a good dehydrating agent. When it reacts with cold conc. H2SO4 it gives a red crystal of chromic(VI) oxide.
Complete step by step answer:
Chromic(VI) oxide that is written as CrO3can be prepared when concentrated sulphuric is added to a saturated solution of potassium dichromate.
Chromic(VI) oxide crystals are red in colour, here in CrO3 the oxidation number of chromium ions is +6.
The balanced chemical equation can be written as when potassium dichromate reacts with cold sulphuric acid is:
K2Cr2O7+Conc.H2SO4→K2SO4+2CrO3+H2O
Here the chromium oxide CrO3 formed is an acidic oxide, therefore it readily reacts with alkalis. It reacts with sodium hydroxide to give sodium chromate and water.The balanced chemical reaction is given as:
CrO3+NaOH→Na2CrO4+H2O
Chromium oxide CrO3 also dissolves in water to yield chromic acid that is H2CrO4. The balanced chemical equation is given as:
CrO3+H2O→H2CrO4
So, the correct answer is Option B.
Note: Potassium dichromate that is K2Cr2O7is an orange solid and it is not soluble in water. It is an analytical reagent and is preferred over sodium dichromate since the latter is hygroscopic in nature. The structure of potassium dichromate consists of two tetrahedral chromate units which are linked together by an oxygen atom.
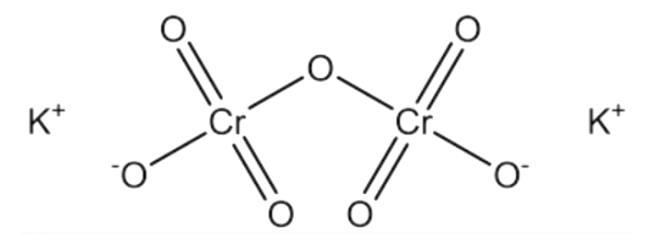
It should be noted that chromium shows an array of oxidation state from II to VI. The highest oxidation state VI however only exists in oxo species like chromium(VI) oxide CrO3.
