Question
Question: Justify that the following reactions are redox reaction: (A) \( CuO(s) + {H_2}(g) \to Cu(s) + {H_2...
Justify that the following reactions are redox reaction:
(A) CuO(s)+H2(g)→Cu(s)+H2O(g)
(B) Fe2O3(s)+3CO(g)→2Fe(s)+3CO2(g)
(C) 4BCl3(g)+3LiAlH4(s)→2B2H6(s)+3LiCl(s)+3AlCl3(s)
(D) 2K(s)+F2(g)→2K+F−(s)
Solution
Hint : The method of determining whether a reaction is a redox reaction or not is simply based upon indicating the oxidation states of each element on the reactant as well as the product side and observing the change in oxidation states associated with loss or gain or electrons.
Complete Step By Step Answer:
Redox reactions are the reactions that involve the simultaneous oxidation and reduction of chemical species. The element that loses electrons is said to be oxidized and the one that gains electrons is said to be reduced.
The copper metal present in the form of its oxide gets reduced on going from a positive two oxidation state to zero oxidation state in the form of copper metal. Hydrogen in its gaseous form is present in zero oxide state and gets oxidized to form water in which it is present in positive one oxidation state. Therefore this reaction can be called a redox reaction.
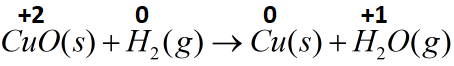
Iron present in the form of its oxide is present in a positive three oxidation state and gets reduced and iron metal with zero oxidation state. Carbon present in carbon monoxide changes its oxidation state from positive two to positive four on forming carbon dioxide. Therefore this reaction can also be classified as a redox reaction.
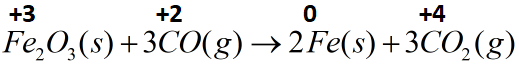
The reaction between boron trichloride and lithium aluminium hydride cannot be classified as a redox reaction as there is no change in the oxidation states of the reactants on forming products. Boron remains in positive three oxidation state in boron trichloride as well and its hydride. The same is true for aluminium.
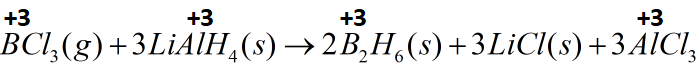
The conversion of potassium metal to a cation and fluorine to fluoride ion that leads to the formation of potassium fluoride (ionic salt) can be classified as a redox reaction as both the reactants are present in zero oxidation state which changes to positive one and negative one for potassium and fluorine respectively.
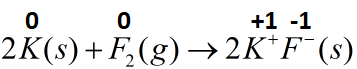
Note :
The numbers written on top of each element in the reaction represents their oxidation states in the particular compound or their elemental form. The change in oxidation states help us visualize the oxidation and reduction reactions taking place simultaneously.
