Question
Question: Select the strongest base in following compound:...
Select the strongest base in following compound:
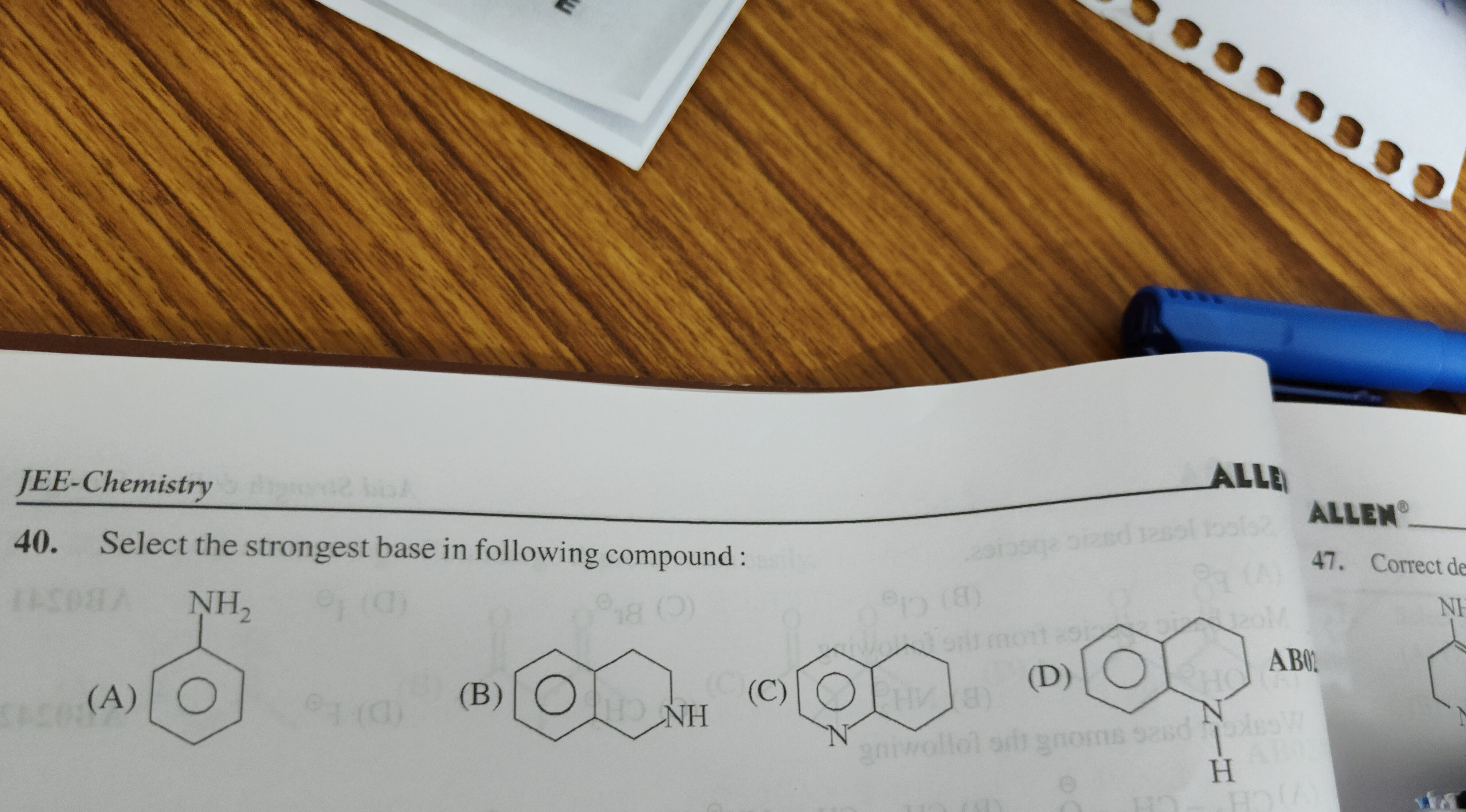
Aniline
1,2,3,4-Tetrahydroquinoline
Quinoline
1,2,3,4-Tetrahydroisoquinoline
1,2,3,4-Tetrahydroisoquinoline
Solution
The basicity of a compound is determined by the availability of the lone pair of electrons on the basic atom (nitrogen in this case) to accept a proton. A stronger base has a more available lone pair, meaning its conjugate acid is less stable.
Let's analyze each compound:
-
(A) Aniline: The nitrogen atom has a lone pair, but it is delocalized into the benzene ring via resonance, reducing its availability for protonation. Thus, aniline is a weak base.
-
(B) 1,2,3,4-Tetrahydroquinoline: The nitrogen is sp3 hybridized and its lone pair is localized. It is more basic than aniline and quinoline because the lone pair is not involved in resonance with the benzene ring.
-
(C) Quinoline: The nitrogen atom is part of an aromatic system, and its lone pair is involved in the aromatic pi system, making it unavailable for protonation. It is a very weak base.
-
(D) 1,2,3,4-Tetrahydroisoquinoline: Similar to (B), the nitrogen is sp3 hybridized and its lone pair is localized.
Comparison: Compounds (B) and (D) are significantly more basic than (A) and (C) because their nitrogen lone pairs are localized. Comparing (B) and (D), empirical data shows that 1,2,3,4-tetrahydroisoquinoline (D) has a lower pKb value (approximately 8.63) compared to 1,2,3,4-tetrahydroquinoline (B) (approximately 8.74). A lower pKb indicates a stronger base. Therefore, (D) is the strongest base.
