Question
Question: 2 NOBr (g) $\rightleftharpoons$ 2 NO (g) + Br₂(g). If nitrosyl bromide (NOBr) is (100/3)% dissociate...
2 NOBr (g) ⇌ 2 NO (g) + Br₂(g). If nitrosyl bromide (NOBr) is (100/3)% dissociated at 25° C and a total pressure of 0.28 atm. Calculate Kₚ for the dissociation at this temperature.
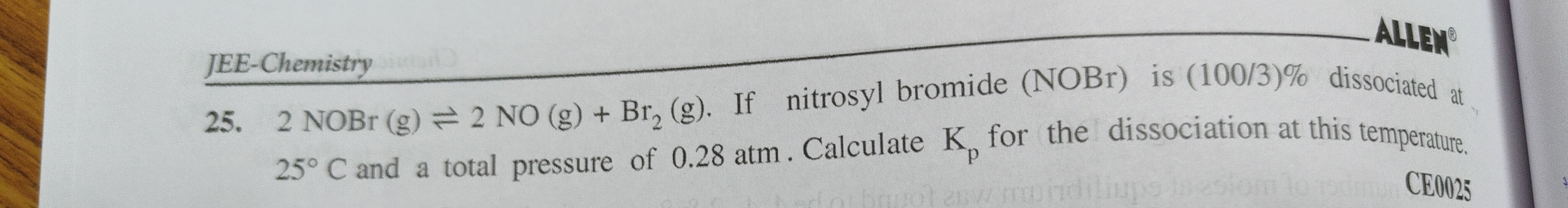
(100/3)% dissociated means α=1/3. Initial pressure: PNOBr=P0 Equilibrium pressures: PNOBr=P0(1−α) PNO=P0α PBr2=P0α/2 Total pressure Ptotal=PNOBr+PNO+PBr2=P0(1−α+α+α/2)=P0(1+α/2) Given Ptotal=0.28 atm and α=1/3. 0.28=P0(1+(1/3)/2)=P0(1+1/6)=P0(7/6) P0=0.28×(6/7)=0.04×6=0.24 atm. Equilibrium partial pressures: PNOBr=0.24(1−1/3)=0.24(2/3)=0.16 atm PNO=0.24(1/3)=0.08 atm PBr2=0.24(1/3)/2=0.24(1/6)=0.04 atm Kp=(PNOBr)2(PNO)2×PBr2=(0.16)2(0.08)2×0.04=0.02560.0064×0.04=0.02560.000256=0.01 atm
0.02 atm
0.005 atm
0.015 atm
0.01 atm
Solution
The reaction is 2NOBr(g)⇌2NO(g)+Br2(g). Let the initial pressure of NOBr be P0. Given the degree of dissociation α=(100/3)%=1/3.
At equilibrium, the partial pressures are: PNOBr=P0(1−α) PNO=P0α PBr2=P0α/2
The total pressure is Ptotal=PNOBr+PNO+PBr2=P0(1−α)+P0α+P0α/2=P0(1+α/2).
Given Ptotal=0.28 atm and α=1/3: 0.28=P0(1+(1/3)/2) 0.28=P0(1+1/6) 0.28=P0(7/6) P0=0.28×(6/7)=0.04×6=0.24 atm.
Now, calculate the equilibrium partial pressures: PNOBr=0.24(1−1/3)=0.24(2/3)=0.16 atm PNO=0.24(1/3)=0.08 atm PBr2=0.24(1/3)/2=0.24(1/6)=0.04 atm
The equilibrium constant Kp is calculated as: Kp=(PNOBr)2(PNO)2×PBr2 Kp=(0.16)2(0.08)2×0.04 Kp=0.02560.0064×0.04 Kp=0.02560.000256=0.01 atm
