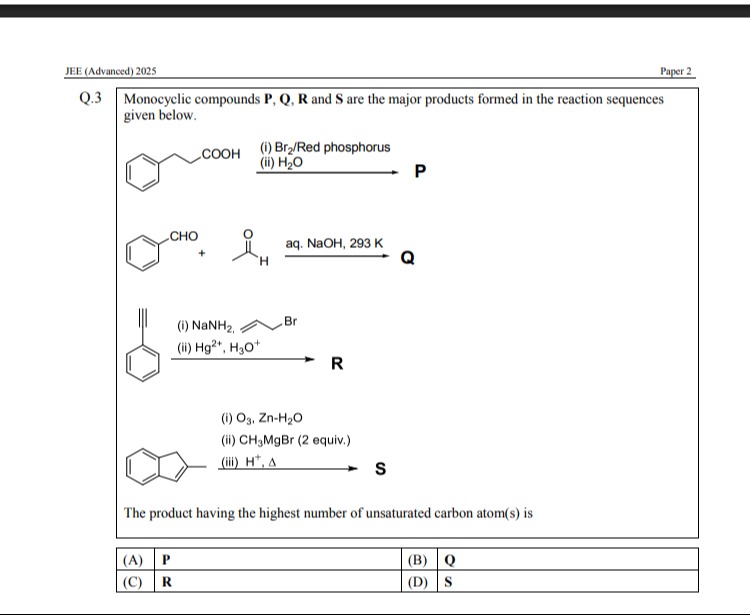
Question
Question: The product having the highest number of unsaturated carbon atom(s) is...
The product having the highest number of unsaturated carbon atom(s) is
P
Q
R
S
Q
Solution
The structures of the products P, Q, R, and S are determined from the given reaction sequences. The number of unsaturated carbon atoms in each product is counted. Unsaturated carbon atoms are those involved in double or triple bonds, including those in aromatic rings.
Product P: The reaction is the Hell-Volhard-Zelinsky (HVZ) reaction on 2-phenylacetic acid. The product is 2-bromo-2-phenylacetic acid. Structure of P: C6H5CH(Br)COOH. Unsaturated carbons: 6 in the phenyl ring + 1 in the carboxyl group (C=O). Total = 7.
Product Q: Aldol condensation between benzaldehyde and acetaldehyde, followed by dehydration. The product is cinnamaldehyde. Structure of Q: C6H5CH=CHCHO. Unsaturated carbons: 6 in the phenyl ring + 2 in the C=C double bond + 1 in the aldehyde group (C=O). Total = 9.
Product R: Phenylacetylene reacts with NaNH2 to form acetylide anion, which then reacts with 1-bromopropane to form 1-phenylpent-1-yne. Hydration of 1-phenylpent-1-yne with Hg2+, H3O+ gives phenyl propyl ketone. Structure of R: C6H5COCH2CH2CH3. Unsaturated carbons: 6 in the phenyl ring + 1 in the carbonyl group (C=O). Total = 7.
Product S: Ozonolysis of indene followed by reaction with excess methylmagnesium bromide and then acid and heat. The final product is a substituted indene derivative. The unsaturated carbons are 6 (benzene ring) + 1 (double bond in the 5 membered ring) = 7.
Therefore, product Q has the highest number of unsaturated carbon atoms.
