Question
Question: Considering the following set of reaction sequences, ...
Considering the following set of reaction sequences,
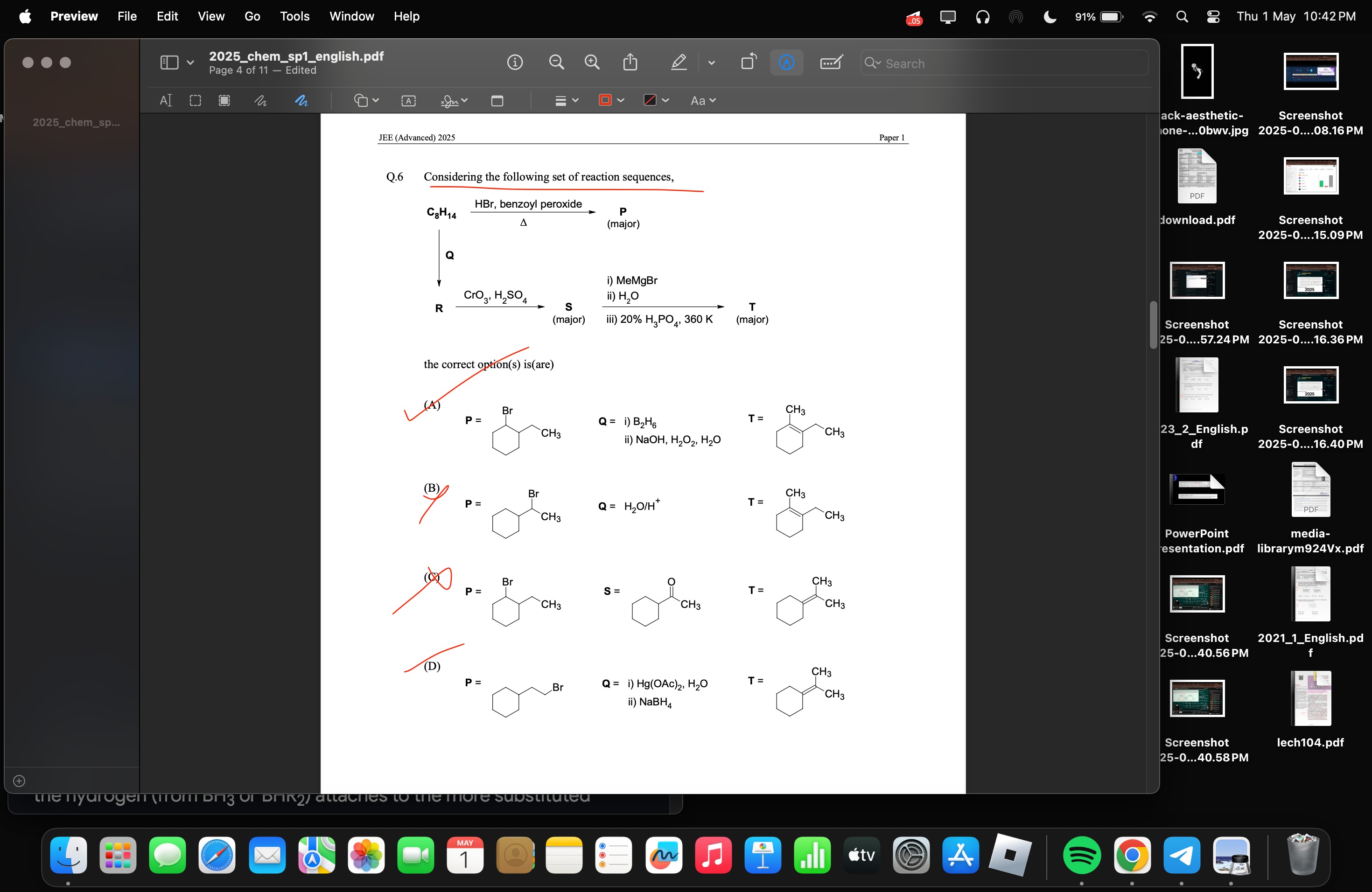
P is
Q is
and T is
P is
Q is
and T is
P is
S is cyclohexyl methyl ketone and T is
P is
and Q is formed by treatment of with .
A
Solution
The question describes a reaction scheme. Let's analyze each option, assuming the starting alkene is 1-methylcyclohexene (C7H12).
-
Option A:
-
P: 1-bromo-2-methylcyclohexane (formed by anti-Markovnikov addition of HBr).
-
Q: 2-methylcyclohexan-1-ol (formed by hydroboration-oxidation, which is anti-Markovnikov addition of water).
-
R: Oxidation of 2-methylcyclohexan-1-ol (a secondary alcohol) yields 2-methylcyclohexanone (S).
-
T: Reaction of 2-methylcyclohexanone with MeMgBr followed by hydrolysis gives 1,2-dimethylcyclohexan-1-ol. Dehydration with 20% H3PO4 at 360 K yields primarily 1,2-dimethylcyclohexene (Saytzeff product).
The structures of P, Q, and T in option A are consistent with the reactions starting from 1-methylcyclohexene.
-
-
Option B:
- Q: Formed by hydration of 1-methylcyclohexene with H2O/H+ (acid-catalyzed hydration, Markovnikov addition). This would yield 1-methylcyclohexan-1-ol (a tertiary alcohol). Tertiary alcohols do not undergo oxidation with Jones reagent. Thus, S is not formed as a ketone. Option B is incorrect.
-
Option C:
- S is given as cyclohexyl methyl ketone. If R is oxidized to S, and S is cyclohexyl methyl ketone, then R must be 1-cyclohexylethan-1-ol (secondary alcohol). If R is 1-cyclohexylethan-1-ol, and it is formed from Q, which is formed from the starting alkene (1-methylcyclohexene), this sequence does not seem consistent. If the starting alkene is 1-methylcyclohexene, Q is 2-methylcyclohexan-1-ol or 1-methylcyclohexan-1-ol. Neither of these upon oxidation gives cyclohexyl methyl ketone. So option C is incorrect.
-
Option D:
- If P is 1-bromo-2-ethylcyclohexane, formed by anti-Markovnikov addition of HBr, the starting alkene must be 1-ethylcyclohexene. However, the subsequent reactions in option D are not consistent with the scheme.
Given the options, it seems the question intends for the starting material to be 1-methylcyclohexene (C7H12), despite stating C8H14. With this assumption, option A is correct.
