Question
Question: The figure displays a fused bicyclic aromatic hydrocarbon. The structure consists of a benzene ring ...
The figure displays a fused bicyclic aromatic hydrocarbon. The structure consists of a benzene ring (a 6-membered ring with alternating double bonds) fused to a cycloheptatriene ring (a 7-membered ring with alternating double bonds). The fusion occurs at two adjacent carbon atoms shared by both rings. This compound is commonly known as Benzocycloheptatriene or Benzo[d]cycloheptatriene. Both the benzene ring and the 7-membered cycloheptatriene ring satisfy Hückel's rule for aromaticity (4n+2 π electrons, where n is an integer) and are planar, contributing to the overall aromatic character of the molecule.
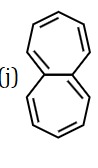
The compound shown is Benzocycloheptatriene. It is an aromatic compound.
Solution
The figure displays a fused bicyclic aromatic hydrocarbon. The structure consists of a benzene ring (a 6-membered ring with alternating double bonds) fused to a cycloheptatriene ring (a 7-membered ring with alternating double bonds). The fusion occurs at two adjacent carbon atoms shared by both rings. This compound is commonly known as Benzocycloheptatriene or Benzo[d]cycloheptatriene. Both the benzene ring and the 7-membered cycloheptatriene ring satisfy Hückel's rule for aromaticity (4n+2 π electrons, where n is an integer) and are planar, contributing to the overall aromatic character of the molecule.
