Question
Question: IUPAC names of the following: (i) \[{{\left( C{{H}_{3}} \right)}_{3}}CC{{H}_{2}}C{{\left( C{{H}_{...
IUPAC names of the following:
(i) (CH3)3CCH2C(CH3)3
(ii) (CH3)2C(C2H5)2
(iii) tetra-tert butylmethane
Solution
According to IUPAC (International Union of Pure and Applied Chemistry), whenever we are going to write the IUPAC name of a compound, we have to give numbering first to functional groups or highly substituted carbon. Means lower numbering should be a functional group or highly substituted carbon present in the molecule or compound.
Complete step by step answer:
-Whenever we are going to write the IUPAC name, we should identify the parent chain or long chain in the given compound.
(CH3)3CCH2C(CH3)3
-We have to write the structure of the given compound (CH3)3CCH2C(CH3)3, then only we can write the IUPAC name.
-The structure of (CH3)3CCH2C(CH3)3 is as follows.
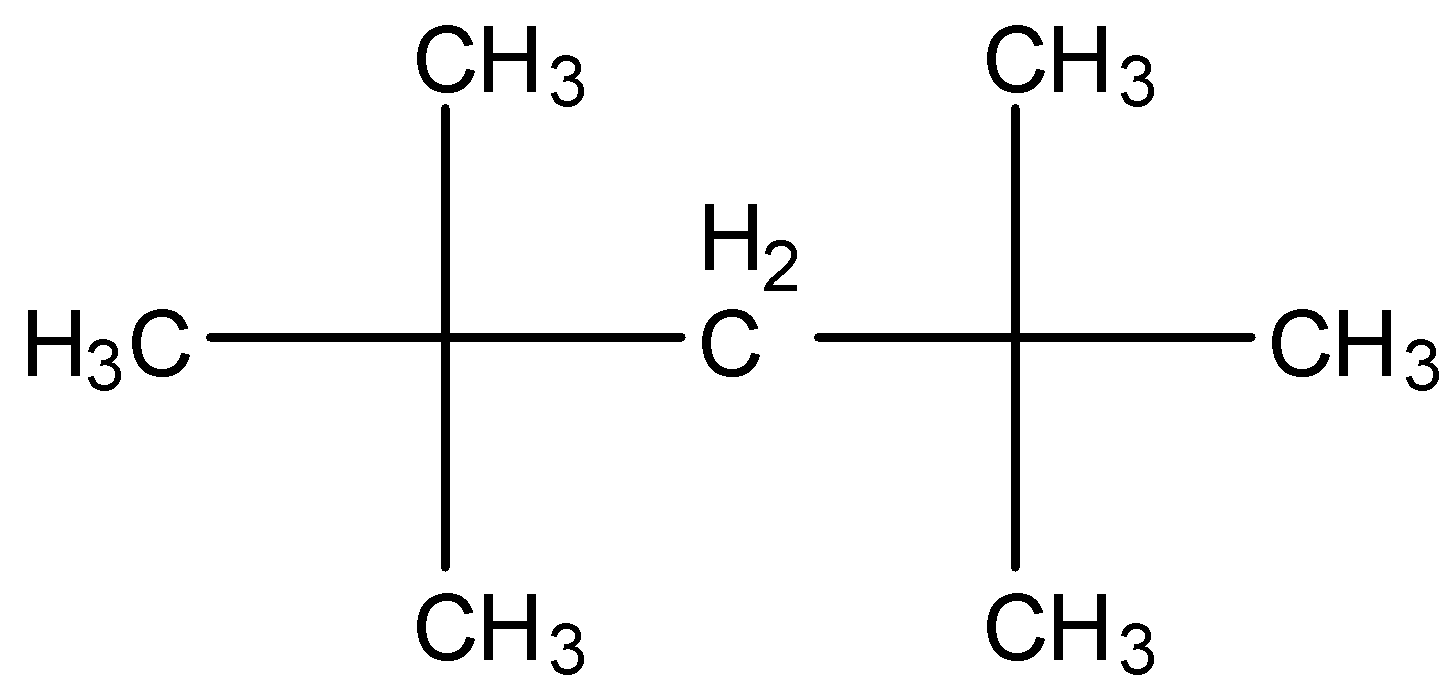
-After knowing the structure of the given compound we have to give the numbering to the longest chain the compound.
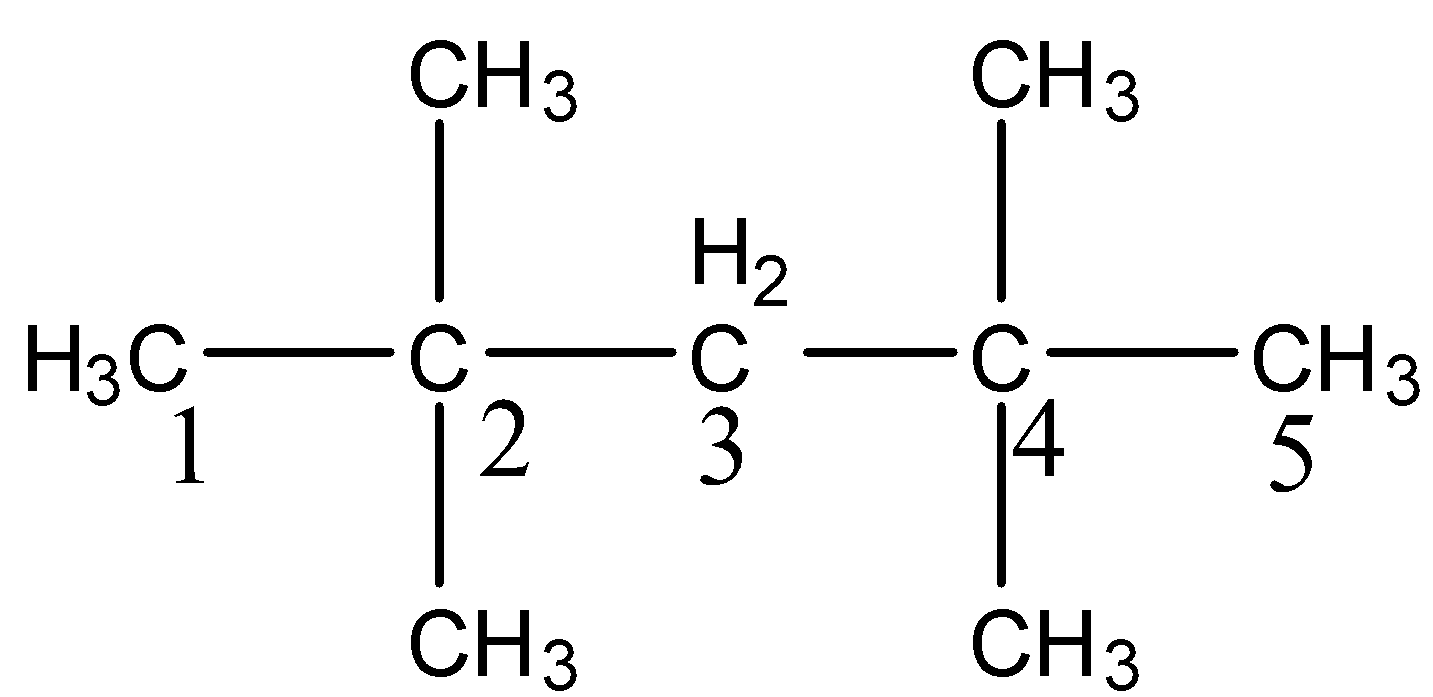
-There are two methyl groups at carbon-2 and two more methyl groups at carbon-4.
-The longest chain has five carbons and four methyl groups as substituents in the above structure.
-Therefore the name of the compound (i) is 2,2,4,4-Tetramethylpentane.
(CH3)2C(C2H5)2
-We have to write the structure of the given compound(CH3)2C(C2H5)2, then only we can write the IUPAC name.
-The structure of (CH3)2C(C2H5)2is as follows.
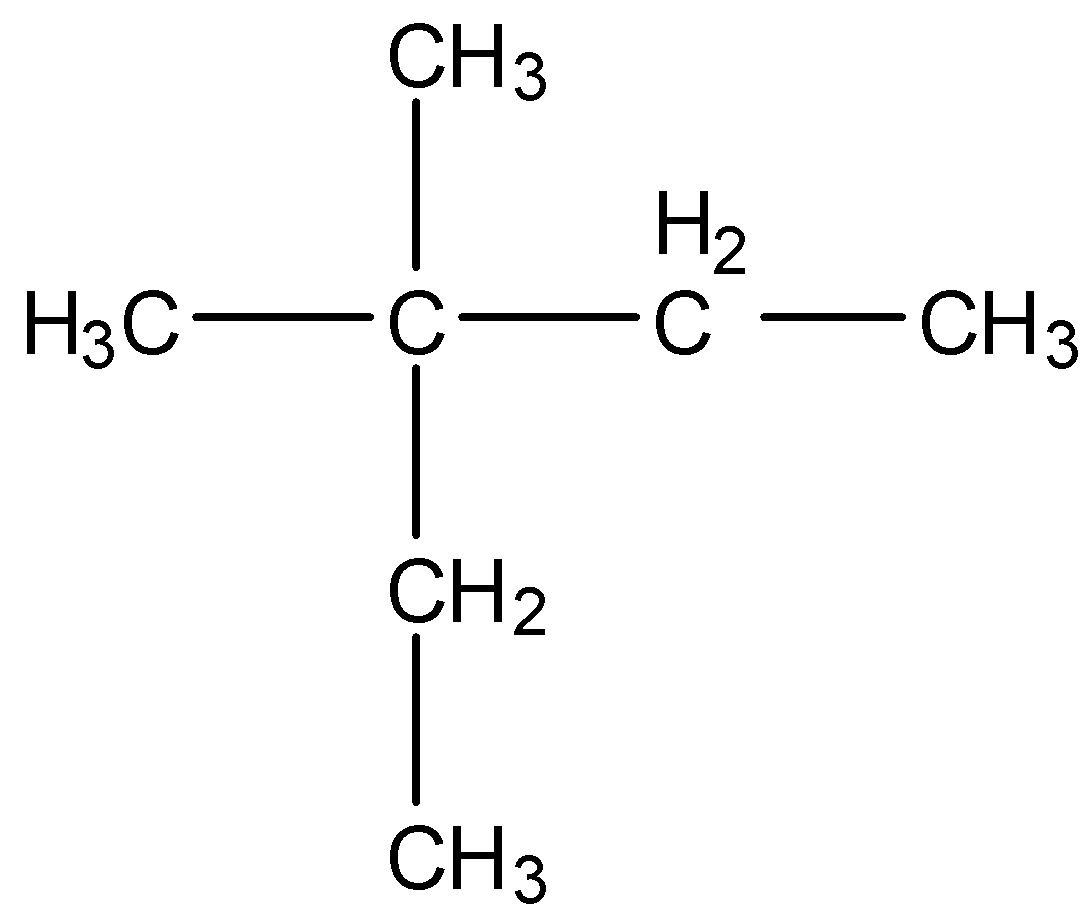
-After knowing the structure of the given compound we have to give numbering to the longest chain of the compound.
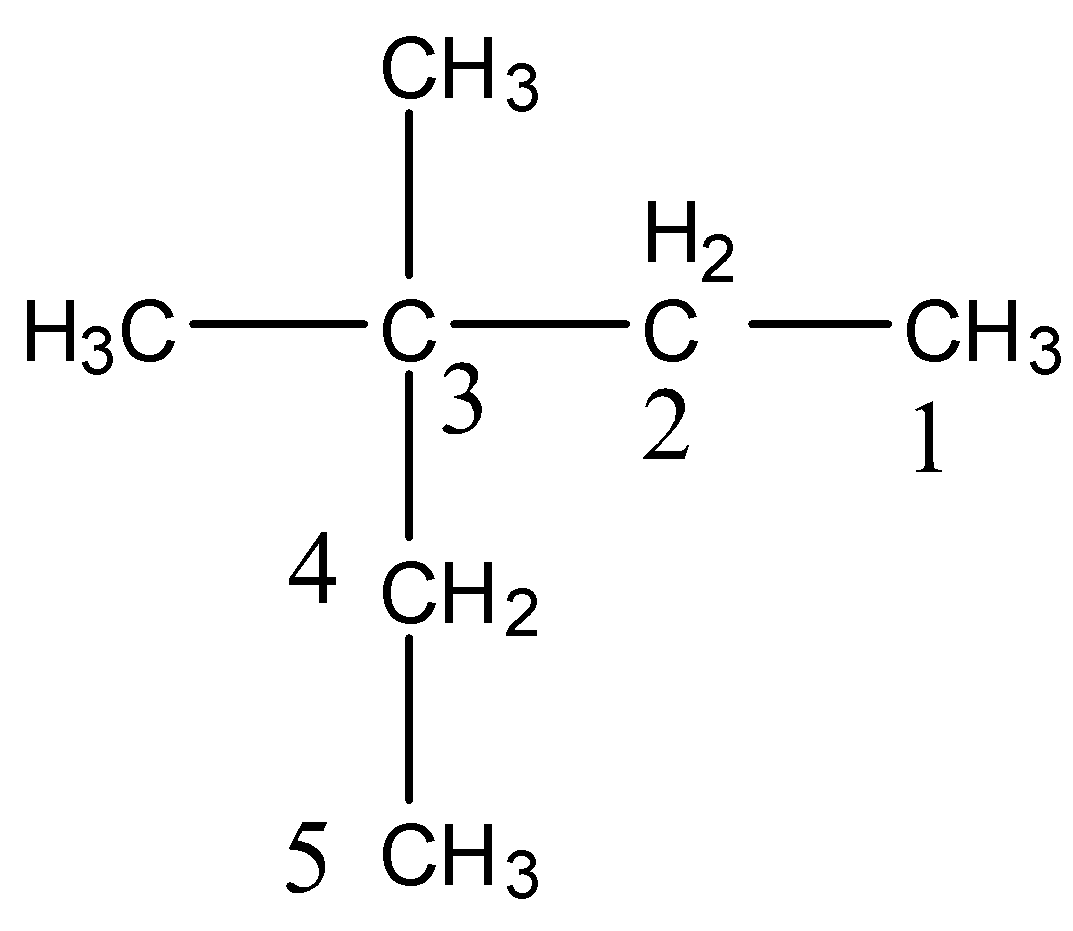
-At Carbon-3 there are two methyl groups attached as substituents.
-The longest chain has five carbons.
-Therefore the name of the compound (ii) is 3,3-Dimethylpentane.
(iii) tetra-tert butylmethane.
-The structure of tetra-tert butylmethane is as follows.
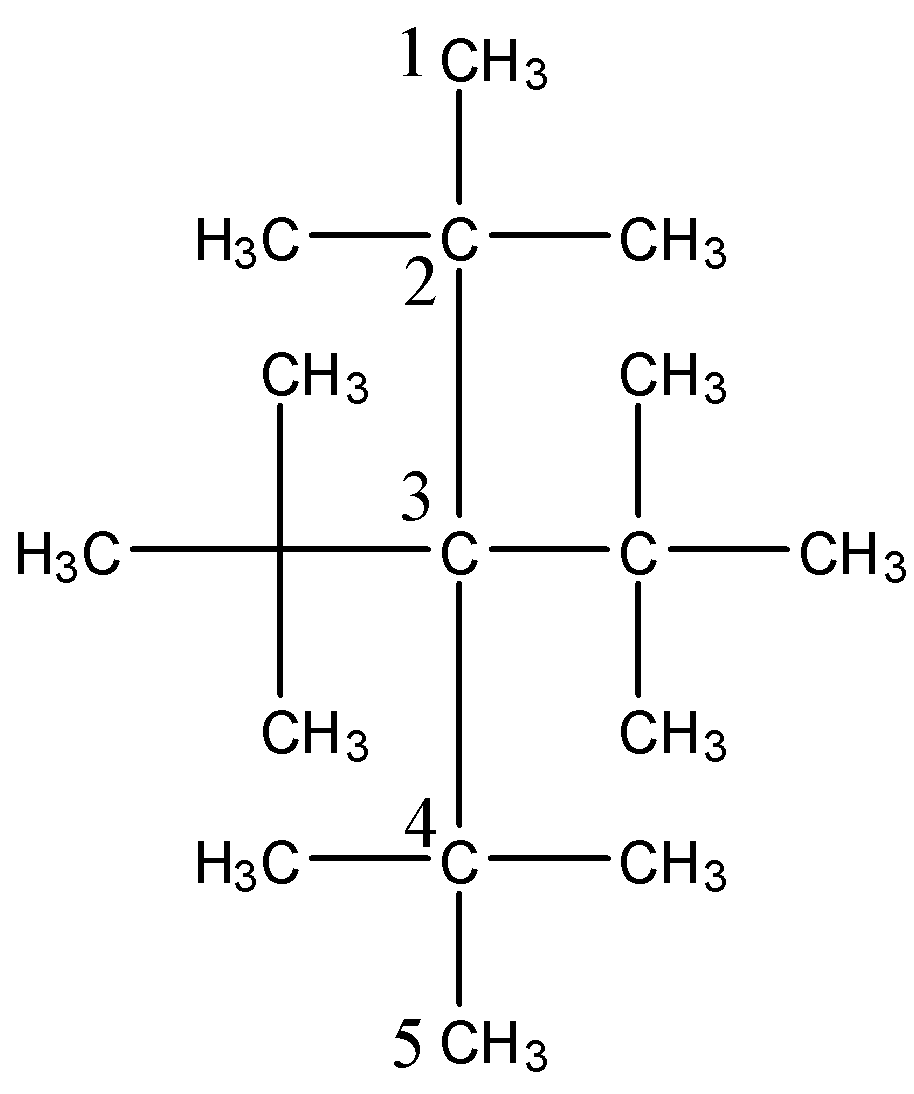
-After knowing the structure of the given compound we have to give numbering to the longest chain of the compound.
-At carbon-2 and Carbon-4 there are two methyl groups attached and at Carbon-3 there are two isopropyl groups attached.
-The IUPAC name of compound (iii) is 3,3-Di-tert-butyl-2,2,4,4-tetramethylpentane.
Note: There are five carbons in the main chain of the all three compounds that is why there is a name given as pentane at the end of the name of all the three compounds. Choosing a parent chain is a crucial step while writing the IUPAC name of the organic compounds.
