Question
Question: IUPAC name of the compound \( C{H_2} = CH - CHO \) is?...
IUPAC name of the compound CH2=CH−CHO is?
Solution
Hint : Here, let us first understand the meaning of the IUPAC nomenclature and then name the given compound. IUPAC is the international organization which provides standardization of atomic units, nomenclature, symbols, etc. of various chemicals. IUPAC has set some of the rules to name the organic or inorganic compounds. These rules are as follows:
-First determine the functional group in the given compound. It will be known as the suffix.
-Now, locate the chain with the required functional group. After locating, add up the number of carbon atoms. This will be the prefix of the given compound.
-We have to identify the senior-most among the given structural compounds joined to the primary characteristic group.
-Specify the unsaturation, if any, after naming the parent hydride.
-Now combine suffix and parent hydride name for determination of the principal characteristic group.
-Then we have to categorize the substituents and then organize them according to the alphabetical order of the corresponding prefix.
-If there are more than one prefixes then add them without altering the current order. Insert locants.
-Now, focus on figuring out the chirality center and other stereogenic units.
At last we have to add up stereo descriptors.
By following these rules of IUPAC we can name the given compound.
Complete Step By Step Answer:
In the given compound CH2=CH−CHO , we have ‘ −CHO ’ as the functional group and it has to be given the first preference according to the rules mentioned above. Now, ‘ = ’ is also the preference and the suffix ‘ −e ’ of the parent alkane to ‘ −al ’. Now, we have to count the no. of carbons in the chain so here on priority the carbon of ‘ −CHO ’ as 1 . Let us see in the figure of the given structure below by considering that we will understand the numbering in the structure and we can also determine the name of the hydride.
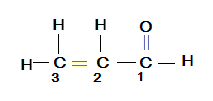
So, according to the count of no. of carbons we know the parent alkane as propane. Also we see that the double bond is attached to the second carbon so we will add ‘ −2 ’ in Prop−2−ene . Now, we know that the suffix has to be replaced by the functional group as this is the priority according to the rule. Thus the given compound is named as:
Prop−2−enal
Here, −e is replaced by −al .
Note :
Here, we have to understand that the parent alkane is propane and because of presence of double bond at the second carbon we named it as Prop−2−ene and then we replaced the suffix as in place of −e as −al . In this way we used the rules of IUPAC and named this structure of the compound.
